10-Q
Q2false--12-310001808158 00-0000000UnlimitedUnlimitedUnlimited Unlimited 2023-11-302025-08-31OneOnehttp://fasb.org/srt/2025#ChiefExecutiveOfficerMember00018081582023-12-310001808158rptx:CollaborationAndLicenseAgreementMemberrptx:HoffmannLaRocheIncAndFHoffmannLaRocheLtdMember2024-01-012024-06-300001808158rptx:TwoThousandTwentyEmployeeSharePurchasePlanMember2024-04-012024-06-300001808158us-gaap:AccumulatedOtherComprehensiveIncomeMember2024-01-012024-03-310001808158us-gaap:FairValueInputsLevel3Memberus-gaap:FairValueMeasurementsRecurringMember2025-06-300001808158country:CA2025-06-300001808158us-gaap:GeneralAndAdministrativeExpenseMemberrptx:RPTXNonEmployeeWarrantExpenseMember2025-04-012025-06-300001808158us-gaap:CommonStockMemberrptx:TwoThousandTwentyEmployeeSharePurchasePlanMember2025-01-012025-03-310001808158us-gaap:AdditionalPaidInCapitalMember2024-04-012024-06-300001808158us-gaap:WarrantMember2024-12-310001808158us-gaap:OperatingSegmentsMember2025-04-012025-06-300001808158us-gaap:RestrictedStockUnitsRSUMember2024-01-012024-06-300001808158us-gaap:EmployeeStockOptionMember2025-01-012025-06-300001808158us-gaap:FairValueMeasurementsRecurringMember2025-06-300001808158us-gaap:RetainedEarningsMember2025-03-310001808158us-gaap:RetainedEarningsMember2024-12-310001808158us-gaap:EmployeeStockOptionMember2025-04-012025-06-300001808158us-gaap:FairValueMeasurementsRecurringMember2024-12-310001808158rptx:CollaborationAndLicenseAgreementMembersrt:MaximumMemberrptx:BristolMyersSquibbCompanyMember2020-05-310001808158us-gaap:RestrictedStockUnitsRSUMember2024-01-012024-06-300001808158country:CH2024-01-012024-06-300001808158rptx:TwoThousandTwentyEquityIncentivePlanMember2024-01-012024-06-300001808158country:US2024-12-310001808158rptx:CollaborationAndLicenseAgreementMemberrptx:HoffmannLaRocheIncAndFHoffmannLaRocheLtdMember2024-02-290001808158rptx:TwoThousandTwentyEmployeeSharePurchasePlanMember2025-01-012025-06-300001808158rptx:TwoThousandTwentyEmployeeSharePurchasePlanMember2025-01-012025-03-310001808158us-gaap:CashAndCashEquivalentsMemberus-gaap:CashMember2025-06-300001808158rptx:DevelopmentCostsMemberus-gaap:OperatingSegmentsMemberrptx:LunresertibProgramMember2025-01-012025-06-300001808158rptx:RPOneSixSixFourProgramMemberrptx:DevelopmentCostsMemberus-gaap:OperatingSegmentsMember2025-04-012025-06-300001808158rptx:CustomerOneMember2025-01-012025-06-300001808158rptx:RPThreeFourSixSevenAndPolProgramMemberrptx:DevelopmentCostsMemberus-gaap:OperatingSegmentsMember2024-04-012024-06-300001808158country:US2025-06-300001808158us-gaap:WarrantMemberrptx:NonEmployeeWarrantExpenseMember2024-11-3000018081582024-01-012024-12-310001808158us-gaap:RestrictedStockUnitsRSUMember2025-06-300001808158rptx:EstimatedSharesIssuableUnderTheEsppMember2025-04-012025-06-300001808158rptx:CollaborationAndLicenseAgreementMemberrptx:HoffmannLaRocheIncAndFHoffmannLaRocheLtdMemberrptx:ThirdAdditionalClinicalDevelopmentPlanMember2023-12-310001808158rptx:DebiopharmMember2025-04-012025-06-300001808158rptx:DevelopmentCostsMemberrptx:RPThreeFourSixSevenAndPolProgramMemberus-gaap:OperatingSegmentsMember2024-01-012024-06-300001808158rptx:CollaborationAndLicenseAgreementMemberrptx:DebiopharmMemberus-gaap:SubsequentEventMember2025-07-152025-07-150001808158rptx:DevelopmentCostsMemberus-gaap:OperatingSegmentsMember2024-01-012024-06-300001808158us-gaap:RetainedEarningsMember2024-04-012024-06-300001808158us-gaap:CommercialPaperMemberus-gaap:FairValueInputsLevel2Memberus-gaap:FairValueMeasurementsRecurringMember2024-12-310001808158rptx:MarketableSecuritiesMemberus-gaap:CommercialPaperMember2025-06-300001808158rptx:TwoThousandTwentyEmployeeSharePurchasePlanMember2020-06-012020-06-300001808158us-gaap:CommonStockMemberrptx:TwoThousandTwentyEmployeeSharePurchasePlanMember2024-01-012024-03-310001808158us-gaap:WarrantMemberrptx:NonEmployeeWarrantExpenseMember2024-11-012024-11-300001808158us-gaap:CashAndCashEquivalentsMemberus-gaap:MoneyMarketFundsMember2024-12-310001808158country:US2025-01-012025-06-300001808158us-gaap:AccumulatedOtherComprehensiveIncomeMember2025-03-310001808158us-gaap:WarrantMember2025-03-310001808158rptx:DevelopmentCostsMemberus-gaap:OperatingSegmentsMemberrptx:CamonsertibProgramMember2025-01-012025-06-300001808158us-gaap:WarrantMember2025-04-012025-06-300001808158rptx:HoffmannLaRocheIncAndFHoffmannLaRocheLtdMember2024-04-012024-06-300001808158us-gaap:FairValueInputsLevel2Memberus-gaap:USTreasurySecuritiesMemberus-gaap:FairValueMeasurementsRecurringMember2024-12-310001808158country:CH2025-01-012025-06-3000018081582024-03-310001808158us-gaap:AdditionalPaidInCapitalMember2024-01-012024-03-310001808158rptx:DebiopharmMember2025-01-012025-06-300001808158us-gaap:AdditionalPaidInCapitalMember2025-01-012025-03-310001808158country:US2024-04-012024-06-300001808158us-gaap:RestrictedStockUnitsRSUMember2025-01-012025-06-300001808158us-gaap:GeneralAndAdministrativeExpenseMemberrptx:RPTXNonEmployeeWarrantExpenseMember2025-01-012025-06-300001808158rptx:DevelopmentCostsMemberrptx:RPOneSixSixFourProgramMemberus-gaap:OperatingSegmentsMember2024-01-012024-06-3000018081582024-04-012024-06-300001808158rptx:TwoThousandTwentyEmployeeSharePurchasePlanMember2025-06-300001808158us-gaap:AdditionalPaidInCapitalMember2025-06-300001808158us-gaap:CommercialPaperMemberus-gaap:FairValueMeasurementsRecurringMember2024-12-310001808158us-gaap:CommercialPaperMemberus-gaap:FairValueInputsLevel2Memberus-gaap:FairValueMeasurementsRecurringMember2025-06-300001808158rptx:TwoThousandTwentyEmployeeSharePurchasePlanMemberus-gaap:AdditionalPaidInCapitalMember2024-01-012024-03-310001808158us-gaap:CashAndCashEquivalentsMember2025-06-300001808158us-gaap:CommonStockMember2025-03-310001808158rptx:EstimatedSharesIssuableUnderTheEsppMember2024-04-012024-06-300001808158us-gaap:CommonStockMember2024-12-310001808158us-gaap:RetainedEarningsMember2024-01-012024-03-3100018081582024-01-012024-06-300001808158rptx:BristolMyersSquibbCompanyMember2024-04-012024-06-300001808158rptx:MarketableSecuritiesMemberus-gaap:CommercialPaperMember2024-12-310001808158us-gaap:FairValueInputsLevel2Memberus-gaap:FairValueMeasurementsRecurringMember2025-06-300001808158us-gaap:AccumulatedOtherComprehensiveIncomeMember2024-04-012024-06-300001808158rptx:DCxBiotherapeuticsCorporationMember2025-05-012025-05-010001808158rptx:TwoThousandTwentyEmployeeSharePurchasePlanMember2024-01-012024-06-300001808158stpr:CA-QC2025-01-012025-06-300001808158us-gaap:GeneralAndAdministrativeExpenseMember2024-01-012024-06-3000018081582025-01-012025-03-310001808158us-gaap:GeneralAndAdministrativeExpenseMember2025-04-012025-06-300001808158us-gaap:OperatingSegmentsMemberrptx:DiscoveryCostsMember2025-04-012025-06-300001808158rptx:EstimatedSharesIssuableUnderTheEsppMember2025-01-012025-06-300001808158us-gaap:MoneyMarketFundsMemberus-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMember2024-12-310001808158us-gaap:GeneralAndAdministrativeExpenseMember2024-04-012024-06-300001808158srt:CumulativeEffectPeriodOfAdoptionAdjustmentMember2025-01-012025-06-3000018081582025-07-310001808158us-gaap:AdditionalPaidInCapitalMember2024-03-310001808158rptx:EstimatedSharesIssuableUnderTheEsppMember2024-01-012024-06-300001808158rptx:OptionsToPurchaseCommonSharesMember2025-01-012025-06-300001808158us-gaap:FairValueInputsLevel3Memberrptx:DCxBiotherapeuticsCorporationMemberrptx:RPTXDilutionProtectionRightsMemberus-gaap:FairValueMeasurementsRecurringMember2025-06-300001808158rptx:TwoThousandTwentyEquityIncentivePlanMember2024-04-012024-06-300001808158us-gaap:CashAndCashEquivalentsMember2024-12-310001808158rptx:RPThreeFourSixSevenAndPolProgramMemberrptx:DevelopmentCostsMemberus-gaap:OperatingSegmentsMember2025-04-012025-06-300001808158rptx:DevelopmentCostsMemberus-gaap:OperatingSegmentsMemberrptx:CamonsertibProgramMember2024-04-012024-06-300001808158us-gaap:CommonStockMember2025-04-012025-06-3000018081582025-04-012025-06-300001808158us-gaap:OperatingSegmentsMember2024-04-012024-06-300001808158us-gaap:AdditionalPaidInCapitalMember2024-12-310001808158rptx:DCxBiotherapeuticsCorporationMember2025-05-010001808158rptx:TwoThousandTwentyEmployeeSharePurchasePlanMemberus-gaap:AdditionalPaidInCapitalMember2025-01-012025-03-3100018081582025-01-012025-06-300001808158rptx:DevelopmentCostsMemberus-gaap:OperatingSegmentsMember2025-04-012025-06-300001808158us-gaap:CashAndCashEquivalentsMemberus-gaap:CommercialPaperMember2024-12-310001808158rptx:BristolMyersSquibbCompanyMember2025-04-012025-06-300001808158us-gaap:RetainedEarningsMember2025-01-012025-03-310001808158us-gaap:OperatingSegmentsMemberrptx:DiscoveryCostsMember2024-01-012024-06-300001808158rptx:TwoThousandTwentyEquityIncentivePlanMember2025-04-012025-06-3000018081582024-12-310001808158rptx:RPTXDilutionProtectionRightsMemberrptx:DCxBiotherapeuticsCorporationMemberus-gaap:FairValueMeasurementsRecurringMember2025-06-300001808158us-gaap:ResearchAndDevelopmentExpenseMember2025-04-012025-06-300001808158country:CH2024-04-012024-06-3000018081582024-08-012024-08-3100018081582025-03-310001808158rptx:CustomerOneMember2025-04-012025-06-300001808158us-gaap:MoneyMarketFundsMemberus-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMember2025-06-300001808158us-gaap:AccumulatedOtherComprehensiveIncomeMember2025-04-012025-06-300001808158us-gaap:CommonStockMember2025-01-012025-03-310001808158country:CA2024-12-310001808158us-gaap:CommonStockMember2024-03-310001808158rptx:MarketableSecuritiesMember2025-06-300001808158rptx:CollaborationAndLicenseAgreementMemberrptx:UndruggableTargetMemberrptx:BristolMyersSquibbCompanyMember2024-03-012024-03-310001808158rptx:TwoThousandTwentyEmployeeSharePurchasePlanMember2025-04-012025-06-300001808158rptx:CommercialMilestonesMemberrptx:CollaborationAndLicenseAgreementMembersrt:MaximumMemberrptx:BristolMyersSquibbCompanyMember2020-05-310001808158us-gaap:RetainedEarningsMember2025-04-012025-06-300001808158us-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMember2024-12-310001808158rptx:CollaborationAndLicenseAgreementMemberrptx:BristolMyersSquibbCompanyMember2025-04-012025-06-300001808158rptx:TwoThousandTwentyEquityIncentivePlanMember2020-06-012020-06-300001808158us-gaap:RetainedEarningsMember2024-03-310001808158country:US2025-04-012025-06-300001808158us-gaap:RestrictedStockUnitsRSUMember2024-04-012024-06-300001808158rptx:OptionsToPurchaseCommonSharesMember2024-04-012024-06-300001808158us-gaap:CommonStockMember2025-06-300001808158rptx:TwoThousandTwentyEmployeeSharePurchasePlanMember2025-01-012025-06-300001808158srt:CumulativeEffectPeriodOfAdoptionAdjustmentMember2025-04-012025-06-300001808158us-gaap:AccumulatedOtherComprehensiveIncomeMember2023-12-310001808158us-gaap:AccumulatedOtherComprehensiveIncomeMember2025-01-012025-03-3100018081582024-01-012024-03-310001808158us-gaap:CashAndCashEquivalentsMemberus-gaap:MoneyMarketFundsMember2025-06-300001808158us-gaap:RestrictedStockUnitsRSUMember2025-04-012025-06-300001808158rptx:OptionsToPurchaseCommonSharesMember2025-04-012025-06-300001808158us-gaap:CorporateDebtSecuritiesMemberus-gaap:FairValueMeasurementsRecurringMember2024-12-310001808158us-gaap:FairValueInputsLevel2Memberus-gaap:FairValueMeasurementsRecurringMember2024-12-310001808158us-gaap:WarrantMember2025-06-300001808158us-gaap:FairValueInputsLevel2Memberus-gaap:CorporateDebtSecuritiesMemberus-gaap:FairValueMeasurementsRecurringMember2024-12-310001808158us-gaap:AdditionalPaidInCapitalMember2024-06-300001808158us-gaap:CarryingReportedAmountFairValueDisclosureMemberrptx:DCxBiotherapeuticsCorporationMember2025-05-010001808158us-gaap:USTreasurySecuritiesMemberus-gaap:FairValueMeasurementsRecurringMember2024-12-310001808158rptx:InducementOptionAwardMember2025-06-300001808158rptx:BristolMyersSquibbCompanyMember2024-01-012024-06-300001808158rptx:ResearchDevelopmentAndRegulatoryMilestonesMemberrptx:CollaborationAndLicenseAgreementMembersrt:MaximumMemberrptx:BristolMyersSquibbCompanyMember2020-05-310001808158us-gaap:MoneyMarketFundsMemberus-gaap:FairValueMeasurementsRecurringMember2024-12-310001808158rptx:MarketableSecuritiesMemberus-gaap:CorporateDebtSecuritiesMember2024-12-310001808158rptx:CollaborationAndLicenseAgreementMemberrptx:BristolMyersSquibbCompanyMember2025-01-012025-06-300001808158rptx:CustomerTwoMember2024-01-012024-06-300001808158us-gaap:RevenueFromContractWithCustomerMemberrptx:CustomerOneMemberus-gaap:CustomerConcentrationRiskMember2025-04-012025-06-300001808158rptx:DevelopmentCostsMemberus-gaap:OperatingSegmentsMemberrptx:LunresertibProgramMember2024-01-012024-06-300001808158rptx:DebiopharmMember2024-04-012024-06-300001808158country:US2024-01-012024-06-300001808158us-gaap:RestrictedStockUnitsRSUMember2025-04-012025-06-3000018081582025-06-300001808158us-gaap:RestrictedStockUnitsRSUMember2024-12-310001808158rptx:CollaborationAndLicenseAgreementMemberrptx:DruggableTargetMemberrptx:BristolMyersSquibbCompanyMember2025-06-012025-06-300001808158us-gaap:RetainedEarningsMember2023-12-310001808158us-gaap:OperatingSegmentsMember2024-01-012024-06-300001808158rptx:CustomerTwoMember2024-04-012024-06-300001808158rptx:CollaborationAndLicenseAgreementMemberrptx:HoffmannLaRocheIncAndFHoffmannLaRocheLtdMemberrptx:ThirdAdditionalClinicalDevelopmentPlanMember2024-03-310001808158rptx:MarketableSecuritiesMember2024-12-310001808158rptx:CustomerTwoMemberus-gaap:RevenueFromContractWithCustomerMemberus-gaap:CustomerConcentrationRiskMember2024-04-012024-06-300001808158us-gaap:OperatingSegmentsMemberrptx:DiscoveryCostsMember2025-01-012025-06-300001808158rptx:DevelopmentCostsMemberus-gaap:OperatingSegmentsMemberrptx:LunresertibProgramMember2025-04-012025-06-300001808158rptx:DevelopmentCostsMemberus-gaap:OperatingSegmentsMember2024-04-012024-06-300001808158us-gaap:CashAndCashEquivalentsMemberus-gaap:CashMember2024-12-310001808158rptx:DevelopmentCostsMemberrptx:RPOneSixSixFourProgramMemberus-gaap:OperatingSegmentsMember2024-04-012024-06-300001808158rptx:TwoThousandTwentyEquityIncentivePlanMember2025-01-012025-06-300001808158rptx:BristolMyersSquibbCompanyMember2025-01-012025-06-300001808158us-gaap:CommercialPaperMemberus-gaap:FairValueMeasurementsRecurringMember2025-06-300001808158us-gaap:RetainedEarningsMember2024-06-300001808158rptx:CollaborationAndLicenseAgreementMemberrptx:DebiopharmMemberus-gaap:SubsequentEventMember2025-07-150001808158us-gaap:EmployeeStockOptionMember2024-04-012024-06-300001808158rptx:DevelopmentCostsMemberrptx:RPThreeFourSixSevenAndPolProgramMemberus-gaap:OperatingSegmentsMember2025-01-012025-06-300001808158rptx:DCxBiotherapeuticsCorporationMember2025-06-300001808158rptx:HoffmannLaRocheIncAndFHoffmannLaRocheLtdMember2024-01-012024-06-300001808158us-gaap:AccumulatedOtherComprehensiveIncomeMember2024-12-310001808158us-gaap:CommonStockMember2024-01-012024-03-310001808158rptx:HoffmannLaRocheIncAndFHoffmannLaRocheLtdMember2025-01-012025-06-300001808158us-gaap:EmployeeStockOptionMember2024-01-012024-06-300001808158rptx:CollaborationAndLicenseAgreementMemberrptx:HoffmannLaRocheIncAndFHoffmannLaRocheLtdMember2024-04-012024-06-300001808158us-gaap:WarrantMember2025-01-012025-03-310001808158rptx:MarketableSecuritiesMemberus-gaap:USTreasurySecuritiesMember2024-12-310001808158us-gaap:RestrictedStockUnitsRSUMember2025-01-012025-06-300001808158us-gaap:OperatingSegmentsMemberrptx:DiscoveryCostsMember2024-04-012024-06-300001808158us-gaap:CommonStockMember2023-12-310001808158us-gaap:OperatingSegmentsMember2025-01-012025-06-300001808158rptx:CollaborationAndLicenseAgreementMemberrptx:HoffmannLaRocheIncAndFHoffmannLaRocheLtdMember2025-04-012025-06-300001808158rptx:TwoThousandTwentyEquityIncentivePlanMember2025-06-300001808158rptx:DebiopharmMember2024-01-012024-06-300001808158us-gaap:ResearchAndDevelopmentExpenseMember2025-01-012025-06-300001808158us-gaap:ResearchAndDevelopmentExpenseMember2024-04-012024-06-300001808158us-gaap:AccumulatedOtherComprehensiveIncomeMember2024-03-310001808158rptx:DevelopmentCostsMemberus-gaap:OperatingSegmentsMemberrptx:CamonsertibProgramMember2025-04-012025-06-300001808158us-gaap:CashAndCashEquivalentsMemberus-gaap:CommercialPaperMember2025-06-300001808158rptx:OptionsToPurchaseCommonSharesMember2024-01-012024-06-300001808158rptx:HoffmannLaRocheIncAndFHoffmannLaRocheLtdMember2025-04-012025-06-300001808158us-gaap:CommonStockMember2024-04-012024-06-300001808158rptx:TwoThousandTwentyEmployeeSharePurchasePlanMember2024-01-012024-03-310001808158rptx:CollaborationAndLicenseAgreementMemberrptx:BristolMyersSquibbCompanyMember2020-05-012020-05-310001808158country:CH2025-04-012025-06-300001808158us-gaap:AccumulatedOtherComprehensiveIncomeMember2024-06-300001808158rptx:DebiopharmMember2025-06-300001808158rptx:CustomerTwoMemberus-gaap:RevenueFromContractWithCustomerMemberus-gaap:CustomerConcentrationRiskMember2024-01-012024-06-300001808158rptx:DevelopmentCostsMemberus-gaap:OperatingSegmentsMember2025-01-012025-06-300001808158rptx:DevelopmentCostsMemberus-gaap:OperatingSegmentsMemberrptx:CamonsertibProgramMember2024-01-012024-06-300001808158us-gaap:RestrictedStockUnitsRSUMember2024-04-012024-06-300001808158us-gaap:AdditionalPaidInCapitalMember2025-04-012025-06-300001808158us-gaap:ResearchAndDevelopmentExpenseMember2024-01-012024-06-300001808158rptx:CollaborationAndLicenseAgreementMemberrptx:HoffmannLaRocheIncAndFHoffmannLaRocheLtdMember2025-01-012025-06-300001808158rptx:CollaborationAndLicenseAgreementMemberrptx:BristolMyersSquibbCompanyMember2024-01-012024-06-3000018081582024-06-300001808158rptx:DevelopmentCostsMemberus-gaap:OperatingSegmentsMemberrptx:LunresertibProgramMember2024-04-012024-06-300001808158rptx:RPOneSixSixFourProgramMemberrptx:DevelopmentCostsMemberus-gaap:OperatingSegmentsMember2025-01-012025-06-300001808158rptx:CollaborationAndLicenseAgreementMemberrptx:BristolMyersSquibbCompanyMember2024-04-012024-06-300001808158us-gaap:AccumulatedOtherComprehensiveIncomeMember2025-06-300001808158us-gaap:RevenueFromContractWithCustomerMemberus-gaap:CustomerConcentrationRiskMemberrptx:CustomerOneMember2025-01-012025-06-300001808158us-gaap:MoneyMarketFundsMemberus-gaap:FairValueMeasurementsRecurringMember2025-06-300001808158us-gaap:AdditionalPaidInCapitalMember2025-03-310001808158us-gaap:CommonStockMember2024-06-300001808158us-gaap:GeneralAndAdministrativeExpenseMember2025-01-012025-06-300001808158us-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMember2025-06-300001808158us-gaap:AdditionalPaidInCapitalMember2023-12-310001808158us-gaap:RetainedEarningsMember2025-06-300001808158us-gaap:EmployeeStockOptionMember2025-06-30xbrli:purerptx:Segmentsxbrli:sharesiso4217:USDrptx:Lease
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, DC 20549
FORM 10-Q
(Mark One)
☒ QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the quarterly period ended June 30, 2025
OR
☐ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the transition period from _________________ to ___________________
Commission File Number: 001-39335
Repare Therapeutics Inc.
(Exact Name of Registrant as Specified in its Charter)
|
|
Québec |
Not applicable |
(State or other jurisdiction of incorporation or organization) |
(I.R.S. Employer
Identification No.) |
7171 Frederick-Banting, Building 2, Suite 270 St-Laurent, Québec, Canada |
H4S 1Z9 |
(Address of principal executive offices) |
(Zip Code) |
Registrant’s telephone number, including area code: (857) 412-7018
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
Title of each class |
|
Trading Symbol(s) |
|
Name of each exchange on which registered |
Common shares, no par value |
|
RPTX |
|
The Nasdaq Stock Market LLC |
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
|
|
|
|
|
|
|
Large accelerated filer |
|
☐ |
|
Accelerated filer |
|
☐ |
|
|
|
|
Non-accelerated filer |
|
☒ |
|
Smaller reporting company |
|
☒ |
|
|
|
|
|
|
|
|
|
|
|
Emerging growth company |
|
☐ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No ☒
As of July 31, 2025, there were 42,959,172 of the registrant’s common shares, no par value per share, outstanding.
SPECIAL NOTE REGARDING FORWARD LOOKING STATEMENTS
This Quarterly Report on Form 10-Q contains forward-looking statements about us and our industry that involve substantial risks and uncertainties. All statements other than statements of historical facts contained in this Quarterly Report on Form 10-Q, including statements regarding our strategy, future financial condition, future operations, research and development costs, plans and objectives of management, are forward-looking statements. In some cases, you can identify forward-looking statements by terminology such as “aim,” “anticipate,” “assume,” “believe,” “contemplate,” “continue,” “could,” “design,” “due,” “estimate,” “expect,” “goal,” “intend,” “may,” “objective,” “plan,” “predict,” “positioned,” “potential,” “seek,” “should,” “target,” “will,” “would” and other similar expressions that are predictions of or indicate future events and future trends, or the negative of these terms or other comparable terminology. Although we believe that we have a reasonable basis for each forward-looking statement contained in this Quarterly Report on Form 10-Q, we caution you that these statements are based on a combination of facts and factors currently known by us and our expectations of the future, about which we cannot be certain.
The forward-looking statements in this Quarterly Report on Form 10-Q include, among other things, statements about:
•the outcome of our strategic review process to identify strategic alternatives and partnering opportunities across our portfolio;
•the impact of our corporate restructuring activities, including with respect to actual and anticipated cost savings and the associated headcount reduction, as well as the potential impacts on employee morale and productivity;
•the initiation, timing, progress and results of our current and future clinical trials and related preparatory work and the period during which the results of the trials will become available;
•our estimates regarding expenses, future revenue, capital requirements and needs for additional financing;
•our ability to obtain regulatory approval of any of our current or future product candidates;
•business disruptions affecting the initiation, patient enrollment, development and operation of our clinical trials, including a public health emergency or pandemic;
•the evolving impact of macroeconomic events on our operations, supply chains, general economic conditions, our ability to raise additional capital, and the continuity of our business, including our preclinical studies and clinical trials, including health pandemics, changes in inflation and foreign exchange rates, the U.S. Federal Reserve raising interest rates, tariffs or other trade barriers, and the Russia-Ukraine and Middle-East conflicts;
•our ability to enroll patients in clinical trials, to timely and successfully complete those trials and to receive necessary regulatory approvals;
•the timing of completion of enrollment and availability of data from our current clinical trials, including ongoing clinical trials of RP-3467 and RP-1664;
•the expected timing of filings with regulatory authorities for any product candidates that we develop;
•our expectations regarding the potential market size and the rate and degree of market acceptance for any current or future product candidates that we develop;
•our ability to receive any milestone or royalty payments under our collaboration and license agreements;
•the effects of competition with respect to our product candidates, as well as innovations by current and future competitors in our industry;
•our ability to fund our working capital requirements;
•our intellectual property position, including the scope of protection we are able to establish, maintain and enforce for intellectual property rights covering our product candidates;
•our financial performance;
•our ability to obtain additional funding for our operations; and
•other risks and uncertainties, including those listed under the section titled “Risk Factors” in this Quarterly Report and elsewhere in our Annual Report on Form 10-K for the fiscal year ended December 31, 2024, filed with the Securities and Exchange Commission, or the SEC, on March 3, 2025.
Although we believe that the expectations reflected in these forward-looking statements are reasonable, these statements relate to our strategy, future operations, future financial position, future revenue, projected costs, prospects, plans, objectives of management and
expected evolution, and involve known and unknown risks, uncertainties and other factors including, without limitation, risks, uncertainties and assumptions regarding the impact of the macroeconomic events on our business, operations, strategy, goals and anticipated timelines, our ongoing and planned preclinical activities, our ability to initiate, enroll, conduct or complete ongoing and planned clinical trials, our timelines for regulatory submissions and our financial position that may cause our actual results, levels of activity, performance or achievements to be materially different from any future results, levels of activity, performance or achievements expressed or implied by these forward-looking statements. You are urged to carefully review the disclosures we make concerning these risks and other factors that may affect our business and operating results in this Quarterly Report on Form 10-Q. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date of this document. Except as required by law, we do not intend, and undertake no obligation, to update any forward-looking information to reflect events or circumstances.
PART I—FINANCIAL INFORMATION
Item 1. Financial Statements.
Repare Therapeutics Inc.
Condensed Consolidated Balance Sheets
(Unaudited)
(Amounts in thousands of U.S. dollars, except share data)
|
|
|
|
|
|
|
|
|
|
|
As of
June 30, |
|
|
As of
December 31, |
|
|
|
2025 |
|
|
2024 |
|
ASSETS |
|
|
|
|
|
|
CURRENT ASSETS: |
|
|
|
|
|
|
Cash and cash equivalents |
|
$ |
67,656 |
|
|
$ |
84,717 |
|
Marketable securities |
|
|
41,816 |
|
|
|
68,074 |
|
Income tax receivable |
|
|
9,922 |
|
|
|
10,600 |
|
Other current receivables |
|
|
4,697 |
|
|
|
1,746 |
|
Prepaid expenses |
|
|
2,481 |
|
|
|
6,012 |
|
Total current assets |
|
|
126,572 |
|
|
|
171,149 |
|
Property and equipment, net |
|
|
72 |
|
|
|
2,294 |
|
Operating lease right-of-use assets |
|
|
629 |
|
|
|
1,924 |
|
Income tax receivable |
|
|
1,029 |
|
|
|
960 |
|
Investment in equity securities |
|
|
1,591 |
|
|
|
— |
|
Other assets |
|
|
600 |
|
|
|
179 |
|
TOTAL ASSETS |
|
$ |
130,493 |
|
|
$ |
176,506 |
|
LIABILITIES AND SHAREHOLDERS’ EQUITY |
|
|
|
|
|
|
CURRENT LIABILITIES: |
|
|
|
|
|
|
Accounts payable |
|
$ |
4,012 |
|
|
$ |
3,623 |
|
Accrued expenses and other current liabilities |
|
|
12,167 |
|
|
|
19,819 |
|
Deferred collaboration cost recovery |
|
|
3,257 |
|
|
|
— |
|
Operating lease liability, current portion |
|
|
649 |
|
|
|
1,845 |
|
Total current liabilities |
|
|
20,085 |
|
|
|
25,287 |
|
Operating lease liability, net of current portion |
|
|
— |
|
|
|
88 |
|
TOTAL LIABILITIES |
|
|
20,085 |
|
|
|
25,375 |
|
SHAREHOLDERS’ EQUITY |
|
|
|
|
|
|
Preferred shares, no par value per share; unlimited shares authorized as of
June 30, 2025 and December 31, 2024; 0 shares issued and outstanding
as of June 30, 2025, and December 31, 2024 |
|
|
— |
|
|
|
— |
|
Common shares, no par value per share; unlimited shares authorized as of
June 30, 2025 and December 31, 2024; 42,959,172 and 42,510,708 shares
issued and outstanding as of June 30, 2025 and December 31, 2024, respectively |
|
|
490,425 |
|
|
|
486,674 |
|
Warrants |
|
|
43 |
|
|
|
10 |
|
Additional paid-in capital |
|
|
84,533 |
|
|
|
82,191 |
|
Accumulated other comprehensive (loss) income |
|
|
(8 |
) |
|
|
54 |
|
Accumulated deficit |
|
|
(464,585 |
) |
|
|
(417,798 |
) |
Total shareholders’ equity |
|
|
110,408 |
|
|
|
151,131 |
|
TOTAL LIABILITIES AND SHAREHOLDERS’ EQUITY |
|
$ |
130,493 |
|
|
$ |
176,506 |
|
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements
Repare Therapeutics Inc.
Condensed Consolidated Statements of Operations and Comprehensive Loss
(Unaudited)
(Amounts in thousands of U.S. dollars, except share and per share data)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended
June 30, |
|
|
Six Months Ended
June 30, |
|
|
|
2025 |
|
|
2024 |
|
|
2025 |
|
|
2024 |
|
Revenue: |
|
|
|
|
|
|
|
|
|
|
|
|
Collaboration agreements |
|
$ |
250 |
|
|
$ |
1,073 |
|
|
$ |
250 |
|
|
$ |
53,477 |
|
Operating expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
Research and development, net of tax credits |
|
|
14,283 |
|
|
|
30,075 |
|
|
|
34,553 |
|
|
|
63,045 |
|
General and administrative |
|
|
6,029 |
|
|
|
8,317 |
|
|
|
13,681 |
|
|
|
16,935 |
|
Restructuring |
|
|
3,384 |
|
|
|
— |
|
|
|
6,649 |
|
|
|
— |
|
Total operating expenses |
|
|
23,696 |
|
|
|
38,392 |
|
|
|
54,883 |
|
|
|
79,980 |
|
Gain on sale of technology and other assets |
|
|
5,666 |
|
|
|
— |
|
|
|
5,666 |
|
|
|
— |
|
Loss from operations |
|
|
(17,780 |
) |
|
|
(37,319 |
) |
|
|
(48,967 |
) |
|
|
(26,503 |
) |
Other income (expense), net |
|
|
|
|
|
|
|
|
|
|
|
|
Realized and unrealized gain on foreign exchange |
|
|
66 |
|
|
|
6 |
|
|
|
64 |
|
|
|
37 |
|
Interest income |
|
|
1,236 |
|
|
|
2,894 |
|
|
|
2,774 |
|
|
|
5,862 |
|
Other expense, net |
|
|
(18 |
) |
|
|
(29 |
) |
|
|
(40 |
) |
|
|
(53 |
) |
Total other income, net |
|
|
1,284 |
|
|
|
2,871 |
|
|
|
2,798 |
|
|
|
5,846 |
|
Loss before income taxes |
|
|
(16,496 |
) |
|
|
(34,448 |
) |
|
|
(46,169 |
) |
|
|
(20,657 |
) |
Income tax expense |
|
|
(248 |
) |
|
|
(326 |
) |
|
|
(618 |
) |
|
|
(955 |
) |
Net loss |
|
$ |
(16,744 |
) |
|
$ |
(34,774 |
) |
|
$ |
(46,787 |
) |
|
$ |
(21,612 |
) |
Other comprehensive loss: |
|
|
|
|
|
|
|
|
|
|
|
|
Unrealized loss on available-for-sale marketable
securities |
|
$ |
(17 |
) |
|
$ |
(21 |
) |
|
$ |
(62 |
) |
|
$ |
(162 |
) |
Total other comprehensive loss |
|
|
(17 |
) |
|
|
(21 |
) |
|
|
(62 |
) |
|
|
(162 |
) |
Comprehensive loss |
|
$ |
(16,761 |
) |
|
$ |
(34,795 |
) |
|
$ |
(46,849 |
) |
|
$ |
(21,774 |
) |
Net loss per share attributable to common shareholders - basic
and diluted |
|
$ |
(0.39 |
) |
|
$ |
(0.82 |
) |
|
$ |
(1.09 |
) |
|
$ |
(0.51 |
) |
Weighted-average common shares outstanding - basic and diluted |
|
|
42,921,936 |
|
|
|
42,445,462 |
|
|
|
42,757,745 |
|
|
|
42,339,732 |
|
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements
Repare Therapeutics Inc.
Condensed Consolidated Statements of Shareholders’ Equity
(Unaudited)
(Amounts in thousands of U.S. dollars, except share data)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common Shares |
|
|
Warrants |
|
|
Additional
Paid-in |
|
|
Accumulated
Other Comprehensive |
|
|
Accumulated |
|
|
Total
Shareholders’ |
|
|
|
Shares |
|
|
Amount |
|
|
|
|
|
Capital |
|
|
Income (Loss) |
|
|
Deficit |
|
|
Equity |
|
Balance, December 31, 2023 |
|
|
42,176,041 |
|
|
$ |
483,350 |
|
|
$ |
— |
|
|
$ |
61,813 |
|
|
$ |
28 |
|
|
$ |
(333,109 |
) |
|
$ |
212,082 |
|
Share-based compensation
expense |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
6,475 |
|
|
|
— |
|
|
|
— |
|
|
|
6,475 |
|
Exercise of stock options |
|
|
8,485 |
|
|
|
27 |
|
|
|
— |
|
|
|
(10 |
) |
|
|
— |
|
|
|
— |
|
|
|
17 |
|
Issuance of common shares
on vesting of restricted
share units |
|
|
200,262 |
|
|
|
2,488 |
|
|
|
— |
|
|
|
(2,488 |
) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
Issuance of common shares
under the 2020 Employee
Share Purchase Plan |
|
|
60,618 |
|
|
|
510 |
|
|
|
— |
|
|
|
(152 |
) |
|
|
— |
|
|
|
— |
|
|
|
358 |
|
Other comprehensive loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(141 |
) |
|
|
— |
|
|
|
(141 |
) |
Net income |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
13,162 |
|
|
|
13,162 |
|
Balance, March 31, 2024 |
|
|
42,445,406 |
|
|
$ |
486,375 |
|
|
$ |
— |
|
|
$ |
65,638 |
|
|
$ |
(113 |
) |
|
$ |
(319,947 |
) |
|
$ |
231,953 |
|
Share-based compensation
expense |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
6,519 |
|
|
|
— |
|
|
|
— |
|
|
|
6,519 |
|
Exercise of stock options |
|
|
127 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
Other comprehensive loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(21 |
) |
|
|
— |
|
|
|
(21 |
) |
Net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(34,774 |
) |
|
|
(34,774 |
) |
Balance, June 30, 2024 |
|
|
42,445,533 |
|
|
$ |
486,375 |
|
|
$ |
— |
|
|
$ |
72,157 |
|
|
$ |
(134 |
) |
|
$ |
(354,721 |
) |
|
$ |
203,677 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, December 31, 2024 |
|
|
42,510,708 |
|
|
$ |
486,674 |
|
|
$ |
10 |
|
|
$ |
82,191 |
|
|
$ |
54 |
|
|
$ |
(417,798 |
) |
|
$ |
151,131 |
|
Share-based compensation
expense |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
3,958 |
|
|
|
— |
|
|
|
— |
|
|
|
3,958 |
|
Issuance of common shares
on vesting of restricted
share units |
|
|
307,456 |
|
|
|
3,002 |
|
|
|
— |
|
|
|
(3,002 |
) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
Issuance of common shares
under the 2020 Employee
Share Purchase Plan |
|
|
73,239 |
|
|
|
160 |
|
|
|
— |
|
|
|
(81 |
) |
|
|
— |
|
|
|
— |
|
|
|
79 |
|
Non-employee warrant
expense |
|
|
— |
|
|
|
— |
|
|
|
17 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
17 |
|
Other comprehensive loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(45 |
) |
|
|
— |
|
|
|
(45 |
) |
Net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(30,043 |
) |
|
|
(30,043 |
) |
Balance, March 31, 2025 |
|
|
42,891,403 |
|
|
$ |
489,836 |
|
|
$ |
27 |
|
|
$ |
83,066 |
|
|
$ |
9 |
|
|
$ |
(447,841 |
) |
|
$ |
125,097 |
|
Share-based compensation
expense |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
2,056 |
|
|
|
— |
|
|
|
— |
|
|
|
2,056 |
|
Issuance of common shares
on vesting of restricted
share units |
|
|
67,769 |
|
|
|
589 |
|
|
|
— |
|
|
|
(589 |
) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
Non-employee warrant
expense |
|
|
— |
|
|
|
— |
|
|
|
16 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
16 |
|
Other comprehensive loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(17 |
) |
|
|
— |
|
|
|
(17 |
) |
Net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(16,744 |
) |
|
|
(16,744 |
) |
Balance, June 30, 2025 |
|
|
42,959,172 |
|
|
$ |
490,425 |
|
|
$ |
43 |
|
|
$ |
84,533 |
|
|
$ |
(8 |
) |
|
$ |
(464,585 |
) |
|
$ |
110,408 |
|
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements
Repare Therapeutics Inc.
Condensed Consolidated Statements of Cash Flows
(Unaudited)
(Amounts in thousands of U.S. dollars)
|
|
|
|
|
|
|
|
|
|
|
Six Months Ended
June 30, |
|
|
|
2025 |
|
|
2024 |
|
Cash Flows From Operating Activities: |
|
|
|
|
|
|
Net loss for the period |
|
$ |
(46,787 |
) |
|
$ |
(21,612 |
) |
Adjustments to reconcile net loss to net cash used in operating activities: |
|
|
|
|
|
|
Share-based compensation expense |
|
|
6,047 |
|
|
|
12,994 |
|
Depreciation expense |
|
|
2,222 |
|
|
|
989 |
|
Non-cash lease expense |
|
|
1,295 |
|
|
|
1,131 |
|
Foreign exchange gain |
|
|
(117 |
) |
|
|
(27 |
) |
Net accretion of marketable securities |
|
|
(1,006 |
) |
|
|
(2,908 |
) |
Gain on sale of technology and other assets |
|
|
(5,666 |
) |
|
|
— |
|
Changes in operating assets and liabilities: |
|
|
|
|
|
|
Prepaid expenses |
|
|
3,006 |
|
|
|
(1,024 |
) |
Other current receivables |
|
|
67 |
|
|
|
910 |
|
Other non-current assets |
|
|
179 |
|
|
|
89 |
|
Accounts payable |
|
|
382 |
|
|
|
4,785 |
|
Accrued expenses and other current liabilities |
|
|
(7,652 |
) |
|
|
(1,731 |
) |
Operating lease liability, current portion |
|
|
(1,223 |
) |
|
|
(401 |
) |
Income taxes |
|
|
609 |
|
|
|
940 |
|
Operating lease liability, net of current portion |
|
|
(88 |
) |
|
|
(765 |
) |
Deferred collaboration cost recovery |
|
|
3,257 |
|
|
|
— |
|
Deferred revenue |
|
|
— |
|
|
|
(11,952 |
) |
Net cash used in by operating activities |
|
|
(45,475 |
) |
|
|
(18,582 |
) |
Cash Flows From Investing Activities: |
|
|
|
|
|
|
Proceeds from sale of technology and other assets |
|
|
1,000 |
|
|
|
— |
|
Proceeds from maturities of marketable securities |
|
|
73,662 |
|
|
|
89,015 |
|
Purchase of marketable securities |
|
|
(46,456 |
) |
|
|
(102,213 |
) |
Net cash provided by (used in) investing activities |
|
|
28,206 |
|
|
|
(13,198 |
) |
Cash Flows From Financing Activities: |
|
|
|
|
|
|
Proceeds from exercise of stock options |
|
|
— |
|
|
|
17 |
|
Proceeds from issuance of common stock under the 2020 Employee Share Purchase Plan |
|
|
79 |
|
|
|
358 |
|
Net cash provided by financing activities |
|
|
79 |
|
|
|
375 |
|
Effect of exchange rate fluctuations on cash held |
|
|
129 |
|
|
|
(43 |
) |
Net Decrease In Cash And Cash Equivalents |
|
|
(17,061 |
) |
|
|
(31,448 |
) |
Cash and cash equivalents at beginning of period |
|
|
84,717 |
|
|
|
111,268 |
|
Cash and cash equivalents at end of period |
|
$ |
67,656 |
|
|
$ |
79,820 |
|
|
|
|
|
|
|
|
Supplemental Disclosure Of Cash Flow Information: |
|
|
|
|
|
|
Non-cash consideration received from sale of technology and other assets
- Investment in equity securities |
|
$ |
1,591 |
|
|
$ |
— |
|
Non-cash consideration received from sale of technology and other assets
- Derivative financial asset |
|
$ |
600 |
|
|
$ |
— |
|
Consideration receivable from sale of technology and other assets
- Other current receivables |
|
$ |
3,000 |
|
|
$ |
— |
|
Contracts transferred to acquirer as part of sale of technology and other assets
- Prepaid expenses |
|
$ |
(525 |
) |
|
$ |
— |
|
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements
REPARE THERAPEUTICS INC.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Amounts in U.S. dollars, unless otherwise specified)
1. Organization and Nature of Business
Repare Therapeutics Inc. (“Repare” or the “Company”) is a precision medicine oncology company focused on the development of synthetic lethality-based therapies for patients with cancer. The Company is governed by the Business Corporations Act (Québec). The Company’s common shares are listed on the Nasdaq Global Select Market under the ticker symbol “RPTX”.
2. Summary of Significant Accounting Policies
Basis of Presentation
The accompanying unaudited condensed consolidated financial statements have been prepared in conformity with generally accepted accounting principles in the United States of America (“U.S. GAAP”). Any reference in these notes to applicable guidance is meant to refer to the authoritative U.S. GAAP as found in the Accounting Standards Codification (“ASC”) and as amended by Accounting Standards Updates (“ASU”) of the Financial Accounting Standards Board (“FASB”).
The unaudited condensed consolidated financial statements have been prepared on the same basis as the audited annual consolidated financial statements as of and for the year ended December 31, 2024, and, in the opinion of management, reflect all adjustments, consisting of normal recurring adjustments, necessary for the fair presentation of the Company’s consolidated financial position as of June 30, 2025, the consolidated results of its operations for the three and six months ended June 30, 2025 and 2024, its statements of shareholders’ equity for the three and six months ended June 30, 2025 and 2024 and its consolidated cash flows for the six months ended June 30, 2025 and 2024.
These unaudited condensed consolidated financial statements should be read in conjunction with the Company’s audited consolidated financial statements and the accompanying notes for the year ended December 31, 2024 included in the Company’s Annual Report on Form 10-K, filed with the Securities and Exchange Commission (the “SEC”) on March 3, 2025 (the “Annual Report”). The condensed consolidated balance sheet data as of December 31, 2024 presented for comparative purposes was derived from the Company’s audited consolidated financial statements but does not include all disclosures required by U.S. GAAP. The results for the three and six months ended June 30, 2025 are not necessarily indicative of the operating results to be expected for the full year or for any other subsequent interim period.
The Company’s significant accounting policies are disclosed in the audited consolidated financial statements for the year ended December 31, 2024 included in the Annual Report. There have been no changes to the Company's significant accounting policies since the date of the audited consolidated financial statements for the year ended December 31, 2024 included in the Annual Report.
Principles of Consolidation
These unaudited condensed consolidated financial statements of the Company include the accounts of the Company and its wholly-owned subsidiary, Repare Therapeutics USA Inc. (“Repare USA”), which was incorporated under the laws of Delaware on June 1, 2017. The financial statements of Repare USA are prepared for the same reporting period as the parent company, using consistent accounting policies. All intra-group transactions, balances, income, and expenses are eliminated in full upon consolidation.
Smaller Reporting Company
Repare continues to qualify as a “smaller reporting company” under the Exchange Act as of June 30, 2025 because the market value of its common shares held by non-affiliates was less than $75 million as of June 30, 2025. As a smaller reporting company, Repare may rely on exemptions from certain disclosure requirements that are available to smaller reporting companies. For so long as the Company remains a smaller reporting company, it is permitted and intends to rely on such exemptions from certain disclosure and other requirements that are applicable to other public companies that are not smaller reporting companies.
Segment Information
Operating segments refer to components of a company that engage in activities for which separate financial information is available and reviewed regularly by the Chief Operating Decision Maker (CODM) in deciding how to allocate resources and assessing performance. The CODM is the Company's Chief Executive Officer and Chief Financial Officer. The Company manages its operations as a single operating segment, which is the research, development and eventual commercialization of precision oncology drugs targeting specific vulnerabilities of tumors in genetically defined patient populations.
Use of Estimates
The preparation of consolidated financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the amounts reported in consolidated financial statements and accompanying notes. Significant estimates and assumptions reflected in these unaudited condensed consolidated financial statements include, but are not limited to, estimates related to revenue recognition, accrued research and development expenses, share-based compensation and income taxes. The Company bases its estimates on historical experience and other market specific or other relevant assumptions that it believes to be reasonable under the circumstances. Actual results could differ from those estimates. Estimates are periodically reviewed in light of changes in circumstances, facts and experience. Changes in estimates are recorded in the period in which they become known.
Recently Issued Accounting Pronouncements Not Yet Adopted
In December 2023, the FASB amended the guidance in ASU 740, Income Taxes, to provide disaggregated income tax disclosures on the rate reconciliation and income taxes paid. The new guidance is effective for public entities in fiscal years beginning after December 15, 2024. Early adoption is permitted. The Company will adopt the new disclosure requirements in its 2025 Annual Report on Form 10-K.
In November 2024, the FASB issued ASU 2024-03, Income Statement - Reporting Comprehensive Income - Expense Disaggregation Disclosures (Subtopic 220-40): Disaggregation of Income Statement Expenses, and issued ASU 2025-01, Income Statement - Reporting Comprehensive Income - Expense Disaggregation Disclosures (Subtopic 220-4): Clarifying the Effective Date in January 2025. These ASUs require public business entities to provide additional disclosures on specific expense categories in the notes to financial statements for both interim and annual reporting periods. While the amendments do not change current disclosure requirements, the amendments change where this information must be presented, requiring certain disclosures to be in a tabular format alongside other disaggregation details. These ASUs are effective for annual periods starting after December 15, 2026, and interim periods after December 15, 2027, with early adoption permitted. The Company is currently evaluating the impact of the adoption of this ASU on its consolidated financial statements.
3. Cash and Cash Equivalents and Marketable Securities
Cash and cash equivalents and marketable securities were comprised of the following:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Amortized Cost |
|
|
Unrealized Gains |
|
|
Unrealized Losses |
|
|
Fair Value |
|
|
|
(in thousands) |
|
As of June 30, 2025 |
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents: |
|
|
|
|
|
|
|
|
|
|
|
|
Cash |
|
$ |
16,195 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
16,195 |
|
Money market funds |
|
|
48,468 |
|
|
|
— |
|
|
|
— |
|
|
|
48,468 |
|
Commercial paper |
|
|
2,993 |
|
|
|
— |
|
|
|
— |
|
|
|
2,993 |
|
Total cash and cash equivalents: |
|
$ |
67,656 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
67,656 |
|
Marketable securities: |
|
|
|
|
|
|
|
|
|
|
|
|
Commercial paper |
|
|
41,824 |
|
|
|
— |
|
|
|
(8 |
) |
|
|
41,816 |
|
Total marketable securities |
|
$ |
41,824 |
|
|
$ |
— |
|
|
$ |
(8 |
) |
|
$ |
41,816 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
As of December 31, 2024 |
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents: |
|
|
|
|
|
|
|
|
|
|
|
|
Cash |
|
$ |
43,762 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
43,762 |
|
Money market funds |
|
|
25,522 |
|
|
|
— |
|
|
|
— |
|
|
|
25,522 |
|
Commercial paper |
|
|
15,430 |
|
|
|
3 |
|
|
|
— |
|
|
|
15,433 |
|
Total cash and cash equivalents: |
|
$ |
84,714 |
|
|
$ |
3 |
|
|
$ |
— |
|
|
$ |
84,717 |
|
Marketable securities: |
|
|
|
|
|
|
|
|
|
|
|
|
U.S. Treasury and government-sponsored
enterprises |
|
$ |
3,013 |
|
|
$ |
1 |
|
|
$ |
— |
|
|
$ |
3,014 |
|
Commercial paper |
|
|
40,688 |
|
|
|
39 |
|
|
|
(1 |
) |
|
|
40,726 |
|
Corporate debt securities |
|
|
24,322 |
|
|
|
13 |
|
|
|
(1 |
) |
|
|
24,334 |
|
Total marketable securities |
|
$ |
68,023 |
|
|
$ |
53 |
|
|
$ |
(2 |
) |
|
$ |
68,074 |
|
Interest receivable was $0.2 million and $0.4 million as of June 30, 2025 and December 31, 2024, respectively, and is included in other current receivables.
Available-for-sale marketable securities with an aggregate fair value of $33.7 million and $7.8 million as of June 30, 2025 and December 31, 2024, respectively, were held in an immaterial, unrealized loss position. These marketable securities have been in an unrealized loss position for less than twelve months. The unrealized losses were not attributed to credit risk but were primarily associated with changes in interest rates and market liquidity. The Company does not intend to sell these securities and it is more likely than not that it will hold these investments for a period of time sufficient to recover the amortized cost. As a result, the Company did not record an allowance for credit losses or other impairment charges for its marketable securities for the six months ended June 30, 2025 and 2024.
The Company recognized nil of net unrealized loss in other comprehensive loss in the three months ended June 30, 2025 and 2024, respectively, and a net unrealized loss of $0.1 million and $0.2 million in the six months ended June 30, 2025 and 2024, respectively, in relation to its cash and cash equivalents and marketable securities.
The maturities of the Company’s marketable securities as of June 30, 2025 and December 31, 2024 are less than one year.
4. Sale of Technology and Other Assets
On May 1, 2025, the Company out-licensed its early-stage discovery platforms, including certain platform and program intellectual property, to DCx Biotherapeutics Corporation (“DCx”). Under the terms of the out-licensing agreement, the Company received a $1.0 million upfront payment and is expected to receive near-term payments of $3.0 million. In addition, the Company received a 9.99% equity position in DCx, including certain dilution protection rights. The Company is eligible to receive potential future out-licensing, clinical and commercial milestone payments, as well as low-single digit tiered sales royalties for the development of certain products by DCx. Additionally, DCx retained some of the Company's preclinical research employees.
As the assets, rights and employees transferred to DCx constitute a business as defined in ASC 805, Business Combinations, the Company accounted for the disposal by applying the derecognition guidance in ASC 810, Consolidation, which requires that a gain or loss be recognized for the difference between the carrying value of the assets sold and the fair value of the consideration received or receivable. As of May 1, 2025, the total fair value of the consideration received or receivable was determined to be $6.2 million, reflecting the $1.0 million upfront payment received, the $3.0 million cash consideration receivable in the near-term, the $1.6 million equity position in DCx (Note 5), and the $0.6 million dilution protection rights (Note 6). The carrying value of assets disposed of as of May 1, 2025 was $0.5 million, reflecting prepaid expenses for R&D services transferred to DCx. All equipment sold and intellectual property out-licensed to DCx had no carrying value as of May 1, 2025. In connection with the disposal, the Company recognized a gain on sale of technology and other assets in the amount of $5.7 million.
Milestones and sales royalties were determined to be contingent consideration by the Company, which will be recognized when amounts are probable and estimable in accordance with ASC 450, Contingencies. No amounts have been recognized as of June 30, 2025.
5. Investment in Equity Securities
Pursuant to the out-licensing agreement entered into with DCx on May 1, 2025 (Note 4), the Company received a $1.6 million 9.99% equity interest in DCx as partial consideration for the transaction. The Company has determined that its equity interest in DCx does not give the Company a controlling financial interest nor significant influence over DCx. Accordingly, the Company has accounted for its equity interest in DCx, a non-publicly traded company, as a financial instrument without a readily determinable fair value. Such interest is recorded at cost on the Company's Condensed Consolidated Balance Sheet, reflecting fair value at closing of the transaction, less impairment, if any, adjusted for observable price changes, in accordance with ASC 321, Investments - Equity.
The initial $1.6 million fair value of the investment was determined using an option-pricing method back-solve approach to estimate DCx's enterprise value and derive an implied equity value for the Company's common share equity interest in DCx from the most recent round of financing completed by DCx, modeling the significant rights and preferences of DCx's preferred and common shares, including, among others, liquidation preferences and conversion rights, and applying market based assumptions for expected term, expected volatility and risk-free rate, as well as affecting a discount for lack of marketability.
The Company evaluates its investment in equity securities for impairment whenever events or changes in circumstances indicate that the carrying amount of such investment may be impaired. If a decline in value of the investment is determined to be other than
temporary, an impairment loss is recognized in Other Income (Expense) in the Consolidated Statement of Operations and Comprehensive Loss. As of June 30, 2025, there has been no change in the carrying amount of the investment.
6. Fair Value Measurements
Financial assets and liabilities carried at fair value are to be classified and disclosed in one of the following three levels of the fair value hierarchy, of which the first two are considered observable and the last is considered unobservable:
•Level 1 – Quoted prices in active markets for identical assets or liabilities.
•Level 2 – Observable inputs (other than Level 1 quoted prices), such as quoted prices in active markets for similar assets or liabilities, quoted prices in markets that are not active for identical or similar assets or liabilities, or other inputs that are observable or can be corroborated by observable market data.
•Level 3 – Unobservable inputs that are supported by little or no market activity that are significant to determining the fair value of the assets or liabilities, including pricing models, discounted cash flow methodologies and similar techniques.
The following table presents information about the Company’s financial assets measured at fair value on a recurring basis and indicates the level of the fair value hierarchy utilized to determine such fair values:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Description |
|
Financial Assets |
|
|
Level 1 |
|
|
Level 2 |
|
|
Level 3 |
|
|
|
(in thousands) |
|
As of June 30, 2025 |
|
|
|
|
|
|
|
|
|
|
|
|
Assets |
|
|
|
|
|
|
|
|
|
|
|
|
Cash equivalents: |
|
|
|
|
|
|
|
|
|
|
|
|
Money market funds |
|
$ |
48,468 |
|
|
$ |
48,468 |
|
|
$ |
— |
|
|
$ |
— |
|
Commercial paper |
|
|
2,993 |
|
|
|
— |
|
|
|
2,993 |
|
|
|
— |
|
Total cash equivalents |
|
|
51,461 |
|
|
|
48,468 |
|
|
|
2,993 |
|
|
|
— |
|
Marketable securities: |
|
|
|
|
|
|
|
|
|
|
|
|
Commercial paper |
|
|
41,816 |
|
|
|
— |
|
|
|
41,816 |
|
|
|
— |
|
Total marketable securities |
|
|
41,816 |
|
|
|
— |
|
|
|
41,816 |
|
|
|
— |
|
Derivative financial asset |
|
|
600 |
|
|
|
— |
|
|
|
— |
|
|
|
600 |
|
Total financial assets |
|
$ |
93,877 |
|
|
$ |
48,468 |
|
|
$ |
44,809 |
|
|
$ |
600 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
As of December 31, 2024 |
|
|
|
|
|
|
|
|
|
|
|
|
Assets |
|
|
|
|
|
|
|
|
|
|
|
|
Cash equivalents: |
|
|
|
|
|
|
|
|
|
|
|
|
Money market funds |
|
$ |
25,522 |
|
|
$ |
25,522 |
|
|
$ |
— |
|
|
$ |
— |
|
Commercial paper |
|
|
15,433 |
|
|
|
— |
|
|
|
15,433 |
|
|
|
— |
|
Total cash equivalents |
|
|
40,955 |
|
|
|
25,522 |
|
|
|
15,433 |
|
|
|
— |
|
Marketable securities: |
|
|
|
|
|
|
|
|
|
|
|
|
U.S. Treasury and government-sponsored enterprises |
|
|
3,014 |
|
|
|
— |
|
|
|
3,014 |
|
|
|
— |
|
Commercial paper |
|
|
40,726 |
|
|
|
— |
|
|
|
40,726 |
|
|
|
— |
|
Corporate debt securities |
|
|
24,334 |
|
|
|
— |
|
|
|
24,334 |
|
|
|
— |
|
Total marketable securities |
|
|
68,074 |
|
|
|
— |
|
|
|
68,074 |
|
|
|
— |
|
Total financial assets |
|
$ |
109,029 |
|
|
$ |
25,522 |
|
|
$ |
83,507 |
|
|
$ |
— |
|
During the six months ended June 30, 2025, there were no transfers between fair value hierarchy levels.
When developing fair value estimates, the Company maximizes the use of observable inputs and minimizes the use of unobservable inputs. When available, the Company uses quoted market prices to measure the fair value. In determining the fair values of cash equivalents and marketable securities at each date presented above, the Company relied on quoted prices for similar securities in active markets or using other inputs that are observable or can be corroborated by observable market data.
Derivative Financial Asset
Pursuant to the out-licensing agreement entered into with DCx on May 1, 2025 (Note 4), anti-dilution protection rights were granted to the Company by DCx under which the Company is entitled to receive additional common shares in DCx, at no cost, guaranteeing the Company's equity interest in DCx remains at 9.99% on a fully-diluted basis until such time as DCx achieves a specified funding threshold. In accordance with ASC 815, Derivatives and Hedging, the Company's anti-dilution protection rights were determined to be freestanding financial instruments that meet the definition of a derivative and have been recorded at fair value within Other Assets on the Company's Condensed Consolidated Balance Sheet, determined according to Level 3 inputs in the fair value hierarchy.
The fair value of the anti-dilution protection rights was established at $0.6 million at inception and at June 30, 2025, using a Monte Carlo simulation methodology, which expresses potential future scenarios that when simulated thousands of times, can be viewed statistically to ascertain fair value, with the initial equity value determined based on DCx's most recent round of financing and probability estimates applied based on the assumed likelihood of future financing rounds.
The Company marks-to-market its anti-dilution protection rights at each reporting period, with changes in fair value recognized in Other Income (Expense) in the Consolidated Statement of Operations and Comprehensive Loss. As at June 30, 2025, there was no change in the fair value of the anti-dilution rights.
7. Other Current Receivables
Other current receivables consisted of the following:
|
|
|
|
|
|
|
|
|
|
|
As of
June 30,
2025 |
|
|
As of
December 31,
2024 |
|
|
|
(in thousands) |
|
Consideration receivable from sale of technology and other assets |
|
$ |
3,000 |
|
|
$ |
— |
|
Research and development tax credits receivable |
|
|
1,026 |
|
|
|
820 |
|
Collaboration revenue receivable |
|
|
250 |
|
|
|
— |
|
Sales tax and other receivables |
|
|
421 |
|
|
|
926 |
|
Total other current receivables |
|
$ |
4,697 |
|
|
$ |
1,746 |
|
8. Accrued Expenses and Other Current Liabilities
Accrued expenses and other current liabilities consisted of the following:
|
|
|
|
|
|
|
|
|
|
|
As of
June 30,
2025 |
|
|
As of
December 31,
2024 |
|
|
|
(in thousands) |
|
Accrued research and development expense |
|
$ |
6,626 |
|
|
$ |
13,360 |
|
Accrued restructuring expenses |
|
|
2,594 |
|
|
|
174 |
|
Accrued compensation and benefits |
|
|
1,878 |
|
|
|
5,617 |
|
Accrued professional services |
|
|
771 |
|
|
|
502 |
|
Other |
|
|
298 |
|
|
|
166 |
|
Total accrued expenses and other current liabilities |
|
$ |
12,167 |
|
|
$ |
19,819 |
|
9. Restructuring Expenses
In August 2024, the Company announced a strategic re-prioritization of the Company's research and development activities to focus its efforts on the advancement of its portfolio of clinical-stage oncology programs. As part of this strategic refocus, the Company reduced its overall workforce by approximately 25%, with a majority of the headcount reductions from the Company’s preclinical group.
In the first quarter of 2025, the Company announced a further re-alignment of resources and a re-prioritization of its clinical portfolio and approved a phased reorganization plan pursuant to which it expects to reduce its workforce by approximately 75% by the fourth quarter of 2025. As a result of this initiative, the Company accelerated the depreciation of its laboratory equipment by $1.0 million and $1.9 million for the three and six months ended June 30, 2025, respectively, reflecting a shorter estimated remaining useful life for the equipment.
For the three and six months ended June 30, 2025, the Company incurred approximately $3.4 million and $6.6 million, respectively, in costs as part of its restructuring efforts (nil for the three and six months ended June 30, 2024), comprised of $2.1 million in severance and termination benefits, $1.0 million in accelerated depreciation expense and $0.3 million in other restructuring charges for the three months ended June 30, 2025, and comprised of $4.4 million in severance and termination benefits, $1.9 million in accelerated depreciation expense and $0.3 million in other restructuring charges for the six months ended June 30, 2025.
10. Collaborative Arrangements
Debiopharm Clinical Study and Collaboration Agreement
In January 2024, the Company entered into a clinical study and collaboration agreement with Debiopharm International S.A. (“Debiopharm”), a privately-owned, Swiss-based biopharmaceutical company, with the aim to explore the synergy between the Company’s compound, lunresertib, and Debiopharm’s compound, Debio 0123, a WEE1 inhibitor (the “Debio Collaboration Agreement”). The Company and Debiopharm collaborated on the development of a combination therapy, with the Company sponsoring the global study, and with all costs related to the collaboration shared equally. The Company and Debiopharm each supplied their respective drugs and retained all commercial rights to their respective compounds, including as monotherapy or as combination therapies. The activities associated with the Debio Collaboration Agreement were coordinated by a joint steering committee, which was comprised of an equal number of representatives from the Company and Debiopharm.
Based on the terms of the Debio Collaboration Agreement, the Company concluded that the Debio Collaboration Agreement met the requirements of a collaboration within the guidance of ASC 808, Collaborative Arrangements, as both parties were active participants in the combination trial and were exposed to significant risks and rewards depending on the success of the combination trial. Accordingly, the net costs associated with the collaboration have been expensed as incurred and recognized within research and development expenses in the condensed consolidated statement of operations and comprehensive loss.
During the three and six months ended June 30, 2025, the Company recognized $1.4 million and $2.7 million, respectively, in net research and development costs with regards to the Debiopharm portion of the 50/50 cost sharing terms under the Debio Collaboration Agreement, and recorded a deferred collaboration cost recovery of $3.3 million as of June 30, 2025, reflecting the acceleration of payments under the Debio Collaboration Agreement in the second quarter of 2025.
During the three and six months ended June 30, 2024 the Company recognized $0.9 million and $1.4 million, respectively, in net research and development costs with regards to the Debiopharm portion of the 50/50 cost sharing terms under the Debio Collaboration Agreement.
As part of the worldwide licensing agreement with Debiopharm signed on July 15, 2025 (Note 16), the Debio Collaboration Agreement was terminated.
11. Revenue Recognition from Collaboration and License Agreements
The following table presents revenue from collaboration and license agreements:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended
June 30, |
|
|
Six Months Ended
June 30, |
|
|
|
2025 |
|
|
2024 |
|
|
2025 |
|
|
2024 |
|
|
|
(in thousands) |
|
|
|
|
|
|
|
|
|
|
Bristol-Myers Squibb Collaboration and License Agreement |
|
$ |
250 |
|
|
$ |
— |
|
|
$ |
250 |
|
|
$ |
2,589 |
|
Roche Collaboration and License Agreement |
|
|
— |
|
|
|
1,073 |
|
|
|
— |
|
|
|
50,888 |
|
Total revenue |
|
$ |
250 |
|
|
$ |
1,073 |
|
|
$ |
250 |
|
|
$ |
53,477 |
|
The Company’s revenue recognition accounting policy, as well as additional information on the Company’s collaboration and license agreements are disclosed in the audited consolidated financial statements for the year ended December 31, 2024 included in the Annual Report.
Bristol-Myers Squibb Collaboration and License Agreement
In May 2020, the Company entered into a collaboration and license agreement (the “BMS Agreement”) with Bristol-Myers Squibb Company (“Bristol-Myers Squibb”), pursuant to which the Company and Bristol-Myers Squibb agreed to collaborate in the research and development of potential new product candidates for the treatment of cancer. The Company provided Bristol-Myers Squibb access to a selected number of its existing screening campaigns and novel campaigns. The Company was responsible for carrying out early-stage research activities directed to identifying potential targets for potential licensing by Bristol-Myers Squibb, in accordance with a mutually agreed upon research plan, and was solely responsible for such costs. The collaboration consisted of programs directed to both druggable targets and to targets commonly considered undruggable to traditional small molecule approaches. Upon Bristol-Myers Squibb’s election to exercise its option to obtain exclusive worldwide licenses for the subsequent development, manufacturing and commercialization of a program, Bristol-Myers Squibb will then be solely responsible for all such worldwide activities and costs.
Although the collaboration term expired in November 2023, the BMS Agreement will not expire until, on a licensed product-by-licensed product and country-by-country basis, the expiration of the applicable royalty term and in its entirety upon expiration of the last royalty term. Either party may terminate earlier upon an uncured material breach of the agreement by the other party, or the insolvency of the other party. Additionally, Bristol-Myers Squibb may terminate the BMS Agreement for any or no reason on a program-by-program basis upon specified written notice.
The Company is entitled to receive up to $301.0 million in total milestones on a program-by-program basis, consisting of $176.0 million in the aggregate for certain specified research, development and regulatory milestones and $125.0 million in the aggregate for certain specified commercial milestones. The Company is further entitled to a tiered percentage royalty on annual net sales ranging from high-single digits to low-double digits, subject to certain specified reductions.
In March 2024, Bristol-Myers Squibb exercised its one remaining option for an undruggable target. As a result, the Company recognized $2.6 million as revenue related to undruggable targets, including the option fee payment of $0.1 million.
In June 2025, the BMS Agreement was amended to enable Bristol-Myers Squibb to exercise an additional option for another druggable target. As a result, the Company recognized $0.3 million as revenue related to druggable targets, reflecting the option fee payment receivable.
The Company recognized $0.3 million and nil for the three months ended June 30, 2025 and 2024, respectively, and $0.3 million and $2.6 million for the six months ended June 30, 2025 and 2024, respectively, as revenue in relation to the BMS Agreement.
Roche Collaboration and License Agreement
In June 2022, the Company entered into a collaboration and license agreement (the “Roche Agreement”) with Hoffmann-La Roche Inc. and F. Hoffmann-La Roche Ltd (collectively, “Roche”) regarding the development and commercialization of the Company’s product candidate camonsertib (also known as RP-3500) and specified other Ataxia-Telangiectasia and Rad3-related protein kinase (“ATR”) inhibitors (the “Licensed Products”). Pursuant to the Roche Agreement, the Company granted Roche a worldwide, perpetual, exclusive, sublicensable license to develop, manufacture, and commercialize the Licensed Products, as well as a non-exclusive, sublicensable license to certain related companion diagnostics. The Company agreed to complete specified ongoing clinical trials in accordance with the development plan in the Roche Agreement, as well as ongoing investigator sponsored trials (together, the
“Continuing Trials”) at the Company’s expense. Roche assumed all subsequent development of camonsertib with the potential to expand development into additional tumors and multiple combination studies.
On February 7, 2024, the Company received a written notice from Roche of their election to terminate the Roche Agreement following a review of Roche’s pipeline and evolving external factors. The termination became effective May 7, 2024, at which time the Company regained global development and commercialization rights for camonsertib from Roche.
In February 2024, the Company received a $40.0 million milestone payment from Roche that was earned upon dosing of the first patient with camonsertib in Roche’s Phase 2 TAPISTRY trial in January 2024.
In March 2024, the Company received a payment of $4.0 million for revisions to the clinical development plan under the Roche Agreement, of which $2.1 million was previously recorded as a receivable at December 31, 2023.
The Company recognized nil and $1.1 million as revenue for the three months ended June 30, 2025 and 2024, respectively, and nil and $50.9 million for the six months ended June 30, 2025 and 2024, respectively, as revenue associated with the Roche Agreement in relation to (i) the $40.0 million milestone achievement in the first quarter of 2024, as well as (ii) the recognition of all remaining deferred revenue for research and development services performed towards the completion of the Continuing Trials during the period.
12. Leases
The Company has historically entered into lease arrangements for its facilities. As of June 30, 2025, the Company had three operating leases with required future minimum payments. The Company’s leases generally do not include termination or purchase options.
In the second quarter of 2025, the Company executed a lease amendment agreement whereby it surrendered its laboratory space under its Montréal, Québec headquarters lease agreement that expires in August 2025. Pursuant to this amendment, the Company vacated the surrendered premises on May 1, 2025 and has no further lease obligations with regards to the surrendered premises. The surrendered premises had been accounted for as a separate contract for lease accounting purposes. In connection with the lease termination, the Company de-recognized its right-of-use asset and lease liability related to the surrendered premises, with a de minimis loss recognized in the three-months ended June 30, 2025.
Operating Leases
The following tables contain a summary of the lease costs recognized under ASC 842 and other information pertaining to the Company’s operating leases:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended
June 30, |
|
|
Six Months Ended
June 30, |
|
|
|
2025 |
|
|
2024 |
|
|
2025 |
|
|
2024 |
|
|
|
(in thousands) |
|
Operating Leases - Lease Costs |
|
|
|
|
|
|
|
|
|
|
|
|
Operating lease costs |
|
$ |
422 |
|
|
$ |
593 |
|
|
$ |
1,012 |
|
|
$ |
1,187 |
|
Short-term lease costs |
|
|
22 |
|
|
|
15 |
|
|
|
56 |
|
|
|
34 |
|
Variable lease costs |
|
|
27 |
|
|
|
83 |
|
|
|
99 |
|
|
|
168 |
|
Total lease costs |
|
$ |
471 |
|
|
$ |
691 |
|
|
$ |
1,167 |
|
|
$ |
1,389 |
|
|
|
|
|
|
|
|
|
|
|
|
Six Months Ended
June 30, |
|
|
|
2025 |
|
|
2024 |
|
|
|
(in thousands, except as specified otherwise) |
|
Other Operating Lease Information |
|
|
|
|
|
|
Operating cash flows used for operating leases |
|
$ |
1,026 |
|
|
$ |
1,223 |
|
Weighted-average remaining lease term (in years) |
|
|
0.55 |
|
|
|
1.00 |
|
Weighted-average discount rate |
|
|
9.9 |
% |
|
|
4.3 |
% |
13. Share-Based Compensation
2020 Employee Share Purchase Plan
In June 2020, the Company’s board of directors adopted, and the Company’s shareholders approved the 2020 Employee Share Purchase Plan (“ESPP”). The number of shares reserved and available for issuance under the ESPP will automatically increase each January 1, beginning on January 1, 2021 and each January 1 thereafter through January 31, 2030, by the lesser of (1) 1.0% of the total number of common shares outstanding on December 31 of the preceding calendar year, (2) 3,300,000 common shares, or (3) such smaller number of common shares as the Company’s board of directors may designate.
The Company issued 73,239 common shares under the ESPP for the six months ended June 30, 2025, at a weighted-average price per share of $1.08, for aggregate proceeds of $0.1 million.
As of June 30, 2025, the number of common shares that may be issued under the ESPP is 2,059,261.
2020 Equity Incentive Plan
In June 2020, the Company’s board of directors adopted, and the Company’s shareholders approved the 2020 Equity Incentive Plan (the “2020 Plan”). The 2020 Plan became effective on the effective date of the Company's initial public offering (the “IPO”), at which time the Company ceased making awards under the Option Plan. The 2020 Plan allows the Company’s compensation committee to make equity-based and cash-based incentive awards to the Company’s officers, employees, directors and consultants including but not limited to stock options and restricted share units. The aggregate number of common shares reserved and available for issuance under the 2020 Plan has automatically increased on January 1 of each year beginning on January 1, 2021 and will continue to increase on January 1 of each year through and including January 1, 2030, by 5% of the outstanding number of common shares on the immediately preceding December 31, or such lesser number of shares as determined by the Company’s board of directors.
As of June 30, 2025, the number of common shares reserved for issuance under the 2020 Plan is 14,507,782.
Inducement Plan
In April 2024, the Company’s board of directors approved the adoption of the 2024 Inducement Plan (the “Inducement Plan”), to be used exclusively for grants of awards to individuals who were not previously employees or directors (or following a bona fide period of non-employment) as a material inducement to such individuals’ entry into employment with the Company, pursuant to Nasdaq Listing Rule 5635(c)(4). The terms and conditions of the Inducement Plan are substantially similar to those of the 2020 Plan.
As of June 30, 2025, the number of common shares that may be issued under the Inducement Plan is 327,800.
Warrants
In November 2024, the Company issued a warrant, as compensation for services to a consultant, to purchase up to 35,000 common shares of the Company at an exercise price of $3.61 per share, vesting in equal quarterly installments over a two-year period. The warrant expires 5 years after the grant date.
During the three and six months ended June 30, 2025, the Company recognized $0.02 million and $0.04 million, respectively, of share-based compensation expense under general and administrative expenses.
Stock Options
The following table summarizes the Company’s stock option activity:
|
|
|
|
|
|
|
|
|
|
|
Number of
shares |
|
|
Weighted
average
exercise price |
|
Outstanding, January 1, 2025 |
|
|
10,883,904 |
|
|
$ |
12.79 |
|
Granted |
|
|
1,950,500 |
|
|
$ |
1.14 |
|
Cancelled or forfeited |
|
|
(2,313,920 |
) |
|
$ |
12.52 |
|
Outstanding, June 30, 2025 |
|
|
10,520,484 |
|
|
$ |
10.69 |
|
The fair value of stock options, and the assumptions used in the Black Scholes option-pricing model to determine the grant date fair value of stock options granted to employees and non-employees were as follows, presented on a weighted average basis:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended
June 30, |
|
|
Six Months Ended
June 30, |
|
|
|
2025 |
|
|
2024 |
|
|
2025 |
|
|
2024 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fair value of stock options |
|
$ |
0.80 |
|
|
$ |
2.67 |
|
|
$ |
0.88 |
|
|
$ |
4.72 |
|
Risk-free interest rate |
|
|
4.01 |
% |
|
|
4.30 |
% |
|
|
4.07 |
% |
|
|
4.21 |
% |
Expected terms (in years) |
|
|
5.50 |
|
|
|
5.33 |
|
|
|
6.28 |
|
|
|
5.97 |
|
Expected volatility |
|
|
91.21 |
% |
|
|
83.57 |
% |
|
|
90.95 |
% |
|
|
83.08 |
% |
Expected dividend yield |
|
|
0.00 |
% |
|
|
0.00 |
% |
|
|
0.00 |
% |
|
|
0.00 |
% |
Restricted Share Units
The following table summarizes the Company’s restricted share unit activity:
|
|
|
|
|
|
|
|
|
|
|
Number of
shares |
|
|
Weighted
average
grant date fair value |
|
Outstanding, January 1, 2025 |
|
|
764,159 |
|
|
$ |
9.22 |
|
Awarded |
|
|
182,000 |
|
|
$ |
1.17 |
|
Vested and released |
|
|
(375,225 |
) |
|
$ |
9.57 |
|
Forfeited |
|
|
(224,849 |
) |
|
$ |
7.18 |
|
Outstanding, June 30, 2025 |
|
|
346,085 |
|
|
$ |
5.93 |
|
The fair value of each restricted share unit is estimated on the date of grant based on the fair value of the Company's common shares on that same date.
Share-Based Compensation
Share-based compensation expense for all awards was allocated as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended
June 30, |
|
|
Six Months Ended
June 30, |
|
|
|
2025 |
|
|
2024 |
|
|
2025 |
|
|
2024 |
|
|
|
(in thousands) |
|
Research and development |
|
$ |
1,018 |
|
|
$ |
3,694 |
|
|
$ |
3,325 |
|
|
$ |
7,113 |
|
General and administrative |
|
|
1,037 |
|
|
|
2,825 |
|
|
|
2,688 |
|
|
|
5,881 |
|
Total share-based compensation expense |
|
$ |
2,056 |
|
|
$ |
6,519 |
|
|
$ |
6,014 |
|
|
$ |
12,994 |
|
Share-based compensation expense by type of award was as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended
June 30, |
|
|
Six Months Ended
June 30, |
|
|
|
2025 |
|
|
2024 |
|
|
2025 |
|
|
2024 |
|
|
|
(in thousands) |
|
Stock options |
|
$ |
1,730 |
|
|
$ |
5,605 |
|
|
$ |
4,455 |
|
|
$ |
11,290 |
|
Restricted share units |
|
|
310 |
|
|
|
803 |
|
|
|
1,511 |
|
|
|
1,512 |
|
ESPP |
|
|
16 |
|
|
|
111 |
|
|
|
48 |
|
|
|
192 |
|
Total share-based compensation expense |
|
$ |
2,056 |
|
|
$ |
6,519 |
|
|
$ |
6,014 |
|
|
$ |
12,994 |
|
The three and six months ended June 30, 2025 include a cumulative-effect adjustment, which reduced overall share-based compensation expense by $0.7 million and $3.6 million, respectively, as a result of the resignation of certain executives during the period. As part of their severance arrangements, the Company approved an acceleration in vesting of their stock option and restricted share unit awards. The Company accounted for the award modifications under ASC 718, Compensation - Stock Compensation, and reflected the decrease in fair value of such modified awards as a cumulative-effect adjustment in the three and six months ended June 30, 2025.
As of June 30, 2025, there was $6.8 million and $1.5 million of unrecognized share-based compensation expense to be recognized over a weighted average period of 1.1 years and 1.3 years related to unvested stock options and unvested restricted share units, respectively.
14. Net Loss per Share
The following table summarizes the computation of basic and diluted net loss per share attributable to common shareholders of the Company:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended
June 30, |
|
|
Six Months Ended
June 30, |
|
|
|
2025 |
|
|
2024 |
|
|
2025 |
|
|
2024 |
|
|
|
(in thousands, except share and per share amounts) |
|
Numerator: |
|
|
|
|
|
|
|
|
|
|
|
|
Net loss |
|
$ |
(16,744 |
) |
|
$ |
(34,774 |
) |
|
$ |
(46,787 |
) |
|
$ |
(21,612 |
) |
Denominator: |
|
|
|
|
|
|
|
|
|
|
|
|
Weighted-average common shares outstanding — basic and
diluted |
|
|
42,921,936 |
|
|
|
42,445,462 |
|
|
|
42,757,745 |
|
|
|
42,339,732 |
|
Net loss per share - basic and diluted |
|
$ |
(0.39 |
) |
|
$ |
(0.82 |
) |
|
$ |
(1.09 |
) |
|
$ |
(0.51 |
) |
The Company excluded the following potential common shares, presented based on amounts outstanding at each period end, from the computation of diluted net loss per share attributable to common shareholders for the periods indicated because including them would have had an anti-dilutive effect:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended
June 30, |
|
|
Six Months Ended
June 30, |
|
|
|
2025 |
|
|
2024 |
|
|
2025 |
|
|
2024 |
|
Options to purchase common shares |
|
|
10,520,484 |
|
|
|
11,288,589 |
|
|
|
10,520,484 |
|
|
|
11,288,589 |
|
Restricted share units |
|
|
346,085 |
|
|
|
871,063 |
|
|
|
346,085 |
|
|
|
871,063 |
|
Estimated shares issuable under the ESPP |
|
|
49,076 |
|
|
|
78,964 |
|
|
|
49,076 |
|
|
|
78,964 |
|
15. Segment information
The Company operates and manages its business as a single reporting and operating segment, which is the research and development of precision oncology drugs targeting specific vulnerabilities of tumors in genetically defined patient populations. The Company's CODM is the Chief Executive Officer and Chief Financial Officer. The CODM assesses performance for the segment and decides how to allocate resources based on consolidated net loss that is reported on the consolidated statement of operations and comprehensive loss. Managing and allocating resources on a consolidated basis enables the CODM to assess the overall level of resources available and how to deploy these resources across functions and programs that are in line with the Company's long-term company-wide strategic goals.
The following table presents reportable segment net loss, including significant expense categories, attributable to the Company's reportable segment for the three and six months ended June 30, 2025 and 2024.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended
June 30, |
|
|
Six Months Ended
June 30, |
|
|
|
2025 |
|
|
2024 |
|
|
2025 |
|
|
2024 |
|
|
|
(in thousands) |
|
Revenue: |
|
|
|
|
|
|
|
|
|
|
|
|
Collaboration agreements |
|
$ |
250 |
|
|
$ |
1,073 |
|
|
$ |
250 |
|
|
$ |
53,477 |
|
Operating expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
Discovery costs |
|
|
|
|
|
|
|
|
|
|
|
|
Direct external costs |
|
|
259 |
|
|
|
1,398 |
|
|
|
957 |
|
|
|
3,124 |
|
Laboratory supplies and research materials |
|
|
90 |
|
|
|
959 |
|
|
|
344 |
|
|
|
1,957 |
|
Personnel related costs |
|
|
243 |
|
|
|
3,232 |
|
|
|
1,750 |
|
|
|
6,418 |
|
Facilities related costs |
|
|
145 |
|
|
|
393 |
|
|
|
503 |
|
|
|
798 |
|
Other costs |
|
|
65 |
|
|
|
842 |
|
|
|
643 |
|
|
|
1,754 |
|
Development costs |
|
|
|
|
|
|
|
|
|
|
|
|
Direct external costs |
|
|
|
|
|
|
|
|
|
|
|
|
Camonsertib program |
|
|
1,848 |
|
|
|
3,961 |
|
|
|
4,114 |
|
|
|
7,941 |
|
Lunresertib program |
|
|
3,999 |
|
|
|
7,660 |
|
|
|
8,274 |
|
|
|
15,767 |
|
RP-1664 program |
|
|
1,579 |
|
|
|
1,412 |
|
|
|
2,921 |
|
|
|
3,008 |
|
RP-3467 and Polθ program |
|
|
1,541 |
|
|
|
773 |
|
|
|
2,638 |
|
|
|
2,328 |
|
Personnel related costs |
|
|
5,070 |
|
|
|
9,186 |
|
|
|
13,233 |
|
|
|
18,845 |
|
Facilities related costs |
|
|
233 |
|
|
|
213 |
|
|
|
480 |
|
|
|
421 |
|
Other costs |
|
|
695 |
|
|
|
1,186 |
|
|
|
1,584 |
|
|
|
2,618 |
|
Debiopharm development cost reimbursement |
|
|
(1,413 |
) |
|
|
(880 |
) |
|
|
(2,682 |
) |
|
|
(1,380 |
) |
R&D tax credits |
|
|
(71 |
) |
|
|
(260 |
) |
|
|
(206 |
) |
|
|
(554 |
) |
Total research and development costs |
|
$ |
14,283 |
|
|
$ |
30,075 |
|
|
$ |
34,553 |
|
|
$ |
63,045 |
|
Personnel related costs |
|
|
2,944 |
|
|
|
5,220 |
|
|
|
7,522 |
|
|
|
10,939 |
|
Other general administrative costs (1) |
|
|
3,085 |
|
|
|
3,097 |
|
|
|
6,159 |
|
|
|
5,996 |
|
Total general and administrative costs |
|
$ |
6,029 |
|
|
$ |
8,317 |
|
|
$ |
13,681 |
|
|
$ |
16,935 |
|
Restructuring costs |
|
|
3,384 |
|
|
|
— |
|
|
|
6,649 |
|
|
|
— |
|
Total operating expenses |
|
$ |
23,696 |
|
|
$ |
38,392 |
|
|
$ |
54,883 |
|
|
$ |
79,980 |
|
Gain on sale of technology and other assets |
|
|
5,666 |
|
|
|
— |
|
|
|
5,666 |
|
|
|
— |
|
Loss from operations |
|
$ |
(17,780 |
) |
|
$ |
(37,319 |
) |
|
$ |
(48,967 |
) |
|
$ |
(26,503 |
) |
Other income, net (2) |
|
|
1,284 |
|
|
|
2,871 |
|
|
|
2,798 |
|
|
|
5,846 |
|
Income tax expense |
|
|
(248 |
) |
|
|
(326 |
) |
|
|
(618 |
) |
|
|
(955 |
) |
Net loss |
|
$ |
(16,744 |
) |
|
$ |
(34,774 |
) |
|
$ |
(46,787 |
) |
|
$ |
(21,612 |
) |
(1) Includes professional fees, directors and officers insurance costs, public company operating costs, information technology related costs, and other administrative costs.
(2) Includes interest income and other expenses.
The following presents segment revenue and long lived assets by geographic location, along with major collaborator information.
Revenue by location is as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended
June 30, |
|
|
Six Months Ended
June 30, |
|
|
|
2025 |
|
|
2024 |
|
|
2025 |
|
|
2024 |
|
|
|
(in thousands) |
|
United States |
|
$ |
250 |
|
|
$ |
— |
|
|
$ |
250 |
|
|
$ |
2,589 |
|
Switzerland |
|
|
— |
|
|
|
1,073 |
|
|
|
— |
|
|
|
50,888 |
|
Total revenue |
|
$ |
250 |
|
|
$ |
1,073 |
|
|
$ |
250 |
|
|
$ |
53,477 |
|
The Company’s property and equipment, net by country of domicile (Canada) and its subsidiary in the United States are as follows:
|
|
|
|
|
|
|
|
|
|
|
As of
June 30, |
|
|
As of
December 31, |
|
|
|
2025 |
|
|
2024 |
|
|
|
(in thousands) |
|
Canada |
|
$ |
— |
|
|
$ |
2,143 |
|
United States |
|
|
72 |
|
|
|
151 |
|
Total property and equipment, net |
|
$ |
72 |
|
|
$ |
2,294 |
|
The Company’s right-of-use assets by country of domicile (Canada) and its subsidiary in the United States are as follows:
|
|
|
|
|
|
|
|
|
|
|
As of
June 30, |
|
|
As of
December 31, |
|
|
|
2025 |
|
|
2024 |
|
|
|
(in thousands) |
|
Canada |
|
$ |
58 |
|
|
$ |
891 |
|
United States |
|
|
571 |
|
|
|
1,033 |
|
Total right-of-use assets, net |
|
$ |
629 |
|
|
$ |
1,924 |
|
Major Customers
The Company had one customer (a major collaborator) that represents more than 10% of total revenue in each of the three and six months ended June 30, 2025. The amount of revenue derived from this customer for the three and six months ended June 30, 2025 was $0.3 million. A different customer (another major collaborator) represented more than 10% of total revenue in each of the three and six months ended June 30, 2024. The amount of revenue derived from this customer for the three and six months ended June 30, 2024 was $1.1 million and $50.9 million, respectively.
16. Subsequent Event
On July 15, 2025, the Company announced that it entered into an exclusive worldwide licensing agreement with Debiopharm regarding its product candidate, lunresertib (also known as RP-6306). Under the terms of the licensing agreement with Debiopharm, the Company will receive a $10 million upfront payment, and is eligible to receive up to $257 million in potential clinical, regulatory, commercial and sales milestones, as well as single-digit royalties on global net sales. Debiopharm will assume sponsorship of the Company's MYTHIC study and will take over existing and future development activities related to lunresertib. This licensing agreement builds upon the existing collaboration between the parties, which was effectively terminated as part of the licensing agreement.
Item 2. Management’s Discussion and Analysis of Financial Condition and Results of Operations.
You should read the following discussion and analysis of our financial condition and results of operations together with (i) our unaudited condensed consolidated financial statements and related notes, appearing elsewhere in this Quarterly Report on Form 10-Q and (ii) the audited consolidated financial statements and related notes and management’s discussion and analysis of financial condition and results of operations for the fiscal year ended December 31, 2024 included in our Annual Report on Form 10-K (the “Annual Report”), filed with the Securities and Exchange Commission, (the “SEC”), on March 3, 2025. Some of the information contained in this discussion and analysis or set forth elsewhere in this Quarterly Report on Form 10-Q, including information with respect to our plans and strategy for our business and related financing, contains forward-looking statements that involve risks and uncertainties. As a result of many factors, including those factors set forth in the “Risk Factors” sections of this Quarterly Report on Form 10-Q and our Annual Report, our actual results could differ materially from the results described in or implied by the forward-looking statements contained in the following discussion and analysis.
Overview
We are a clinical-stage precision oncology company enabled by our proprietary synthetic lethality approach to the discovery and development of novel therapeutics. Synthetic lethality (SL) represents a clinically validated approach to drug development. We have developed highly targeted cancer therapies focused on genomic instability, including DNA damage repair. SL arises when a deficiency in either of two genes is tolerated in cells, but simultaneous deficiencies in both genes cause cell death. Cancer cells that contain a mutation in one gene of a SL pair are susceptible to therapeutic intervention targeting the other gene pair.
Strategic Re-Prioritization
In January 2025, we announced a re-alignment of resources and a re-prioritization of our clinical portfolio to focus on the continued advancement of our Phase 1 clinical programs, RP-3467 and RP-1664.
On February 24, 2025, we approved a phased reorganization to reduce our workforce by approximately 75% by the fourth quarter of 2025, with our remaining employees primarily focused on the continued advancement of our Phase 1 clinical programs, RP-3467 and RP-1664. In May 2025, we announced the out-licensing of our discovery platforms to DCx Biotherapeutics Corporation, or DCx, and in July 2025, we announced the an exclusive licensing agreement with Debiopharm International S.A., or Debiopharm, for lunresertib.
We continue to actively explore a full range of strategic alternatives, partnerships and sale opportunities across our portfolio to maximize shareholder value.
Our Pipeline
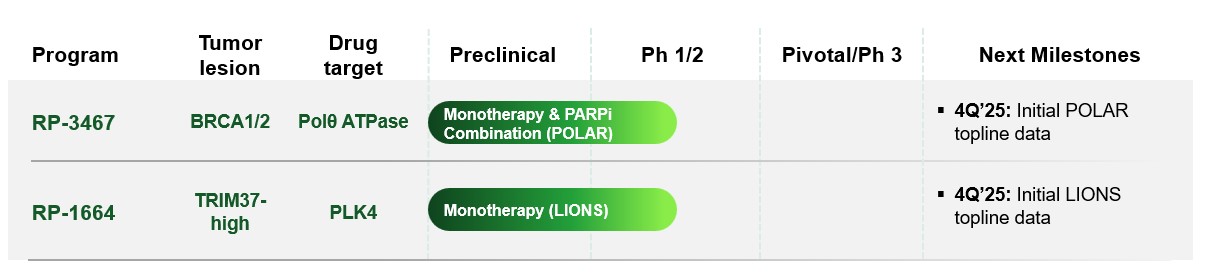
•RP-3467 - We are conducting a Phase 1 clinical trial of RP-3467 (POLAR), dosing patients alone and in combination with the poly-ADP ribose polymerase (PARP) inhibitor, olaparib. POLAR is a multi-center, open-label, dose-escalation Phase 1 clinical trial designed to investigate the safety, pharmacokinetics, pharmacodynamics, and preliminary clinical activity of RP-3467 alone or in combination with olaparib in adults with locally advanced or metastatic epithelial ovarian cancer, metastatic breast cancer, metastatic castration-resistant prostate cancer, or pancreatic adenocarcinoma.
oUpcoming expected milestone:
▪Q4-2025 - Topline safety, tolerability and early efficacy data from the POLAR trial in monotherapy and in combination with olaparib.
•RP-1664 - We completed enrolment of 29 patients in our Phase 1 LIONS clinical trial evaluating RP-1664 as a monotherapy in adult and adolescent patients with TRIM37-high solid tumors. LIONS is a first-in-human, multi-center, open-label Phase 1 clinical trial designed to investigate safety, pharmacokinetics, pharmacodynamics and the preliminary efficacy of RP-1664.
oUpcoming expected milestone:
▪Q4-2025 - Initial topline safety, tolerability and early efficacy data from the LIONS trial
Recent Developments
•Worldwide licensing agreement with Debiopharm for lunresertib
oIn July 2025, we entered into an exclusive worldwide licensing agreement with Debiopharm for lunresertib, a first-in-class oncology PKMYT1 inhibitor. Under the terms of the agreement, Repare will receive a $10 million upfront payment, and is eligible to receive up to $257 million in potential clinical, regulatory, commercial and sales milestones, and single-digit royalties on global net sales. This agreement builds on the success of Repare and Debiopharm's clinical study and collaboration agreement to explore the synergy between lunresertib and Debio 0123, a potential best-in-class, brain penetrant and highly selective WEE1 inhibitor. Debiopharm will assume the sponsorship of the MYTHIC study and will take over existing and future development activities related to lunresertib.
•Out-licensing of our discovery platforms to DCx
oIn May 2025, we announced that we out-licensed our early-stage discovery platforms, including certain platform and program intellectual property, to DCx. Under the terms of the out-licensing agreement, we received a $1 million upfront payment and are expecting to receive $3 million in near-term payments. In addition, we received a 9.99% equity position in DCx, including certain dilution protection rights, and are eligible to receive potential future out-licensing, clinical and commercial milestone payments, as well as low single-digit tiered sales royalties for the development of certain products by DCx. In connection with this transaction, we recognized a $5.7 million gain during the quarter.
•Amendment to our collaboration and license agreement with Bristol-Myers Squibb Company to include additional druggable target in the collaboration
oWe recognized $0.3 million during the quarter as revenue related to druggable targets, reflecting the option fee payment.
Liquidity Overview
As of June 30, 2025, we had cash and cash equivalents and marketable securities on hand of $109.5 million. We believe that our cash, cash equivalents, and marketable securities will be sufficient to fund our anticipated operating and capital expenditure requirements through 2027, after taking into account the re-alignment of resources, reduction in workforce and out-licensing transactions with Debiopharm and DCx. We have based this estimate on assumptions that may prove to be wrong, and we could exhaust our capital resources sooner than we expect.
Since inception, we have incurred significant operating losses. Our net losses were $84.7 million and $93.8 million for the years ended December 31, 2024 and 2023, respectively, and $46.8 million for the six months ended June 30, 2025. As of June 30, 2025, we had an accumulated deficit of $464.6 million.
We expect to continue to incur significant expenses and operating losses for the foreseeable future, as we advance our product candidates through clinical development and seek regulatory approvals, manufacture drug product and drug supply, as well as maintain and expand our intellectual property portfolio. For additional information regarding our liquidity, see the section titled “Liquidity and Capital Resources.”
Macroeconomic Considerations and Other Global Uncertainties
Unfavorable conditions in the economy in the United States, Canada and abroad may negatively affect the growth of our business and our results of operations. For example, macroeconomic events, including health pandemics, changes in inflation and interest rates as well as foreign currency exchange rates, global trade restrictions and the potential imposition of tariffs, natural disasters, supply chain disruptions and the Russia-Ukraine and Middle-East conflicts, have led to economic uncertainty globally and could impact our overall
business operations. The effect of macroeconomic conditions may not be fully reflected in our results of operations until future periods. If, however, economic uncertainty increases or the global economy worsens, our business, financial condition and results of operations may be harmed.
In addition, because some of our manufacturers and suppliers are located in China, we are exposed to the possibility of clinical supply disruption and increased costs in the event of changes in the policies, laws, rules and regulations of the United States or Chinese governments, as well as political unrest or unstable economic conditions in China. For example, trade tensions between the Unites States and China have been escalating in recent years. Most notably, several rounds of U.S. tariffs have been placed on Chinese goods being exported to the United States by the U.S. government. Each of these U.S. tariff impositions against Chinese exports was followed by a round of a retaliatory tariffs by the Chinese government on U.S. exports to China. While our clinical supply has not been affected by these tariffs to date, our components may in the future be subject to these and additional tariffs, which could increase our manufacturing costs and could make our products, if successfully developed and approved, less competitive than those of our competitors whose inputs are not subject to these tariffs. We may otherwise experience supply disruptions or delays, and although we carefully manage our supply and lead-times, our suppliers may not continue to provide us with clinical supply in our required quantities, to our required specifications and quality levels or at attractive prices. In addition, certain Chinese biotechnology companies and CMOs may become subject to trade restrictions, sanctions, other regulatory requirements, or proposed legislation by the U.S. government, which could restrict or even prohibit our ability to work with such entities, thereby potentially disrupting the supply of material to us. Such disruption could have adverse effects on the development of our product candidates and our business operations.
For further discussion of the potential impacts of macroeconomic events on our business, financial condition, and operating results, see the section titled “Risk Factors” elsewhere in this Quarterly Report and in our Annual Report on Form 10-K for the year ended December 31, 2024, filed with the SEC.
Components of Results of Operations
Revenue
To date, we have not recognized any revenue from product sales, and we do not expect to generate any revenue from the sale of products in the foreseeable future. If our development efforts for our product candidates are successful and result in regulatory approval, or license agreements with third parties, we may generate revenue in the future from product sales. However, there can be no assurance as to when we will generate such revenue, if at all.
The following table presents revenue from our collaboration agreements:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended
June 30, |
|
|
Six Months Ended
June 30, |
|
|
|
2025 |
|
|
2024 |
|
|
2025 |
|
|
2024 |
|
|
|
(in thousands) |
|
|
|
|
|
|
|
|
|
|
Bristol-Myers Squibb Collaboration and License Agreement |
|
$ |
250 |
|
|
$ |
— |
|
|
$ |
250 |
|
|
$ |
2,589 |
|
Roche Collaboration and License Agreement |
|
|
— |
|
|
|
1,073 |
|
|
|
— |
|
|
|
50,888 |
|
Total revenue |
|
$ |
250 |
|
|
$ |
1,073 |
|
|
$ |
250 |
|
|
$ |
53,477 |
|
Collaboration and License Agreement with Bristol-Myers Squibb Company
In May 2020, we entered into a collaboration and license agreement, or the BMS Agreement, with the Bristol-Myers Squibb Company, or Bristol-Myers Squibb, pursuant to which we and Bristol-Myers Squibb agreed to collaborate in the research and development of potential new product candidates for the treatment of cancer. The collaboration consisted of programs directed to both druggable targets and to targets commonly considered undruggable to traditional small molecule approaches.
In March 2024, Bristol-Myers Squibb exercised its one remaining option for an undruggable target for a combined total of five druggable targets and one undruggable target over the course of the collaboration. As a result, we recognized the remaining deferred revenue of $2.6 million as revenue related to undruggable targets, including an option fee payment of $0.1 million.
In June 2025, the BMS Agreement was amended to enable Bristol-Myers Squibb to exercise an additional option for another druggable target. As a result, we recognized $0.3 million as revenue related to druggable targets in the second quarter of 2025, reflecting the option fee payment.
We recognized as revenue $0.3 million and nil for the three months ended June 30, 2025 and June 30, 2024, respectively, and $0.3 million and $2.6 million for the six months ended June 30, 2025 and 2024, respectively.
Collaboration and License Agreement with Hoffmann-La Roche Inc. and F. Hoffmann-La Roche Ltd
On June 1, 2022, we entered into a collaboration and license agreement, or the Roche Agreement, with Roche regarding the development and commercialization of our product candidate camonsertib (also known as RP-3500) and specified other ATR inhibitors, which we referred to as the Licensed Products.
We recognized $50.9 million for the six months ended June 30, 2024 as revenue associated with the Roche Agreement in relation to (i) the recognition of revenue upon a $40.0 million milestone achievement in the first quarter of 2024, as well as (ii) the full recognition of deferred revenue for research and development services performed towards the completion of the Continuing Trials during the period.
On February 7, 2024, we received a written notice from Roche of their election to terminate the Roche Agreement following a review of Roche’s pipeline and evolving external factors. The termination became effective May 7, 2024, at which time we regained global development and commercialization rights for camonsertib from Roche.
As of December 31, 2024, all revenue associated with the Roche Agreement was recognized as the related performance obligations were fully satisfied.
Operating Expenses
Debiopharm Collaborative Arrangement
In January 2024, we entered into a clinical study and collaboration agreement, or the Debio Collaboration Agreement, with Debiopharm International S.A., or Debiopharm, a privately-owned, Swiss-based biopharmaceutical company, with the aim to explore the synergy between our compound, lunresertib, and Debiopharm’s compound, Debio 0123, a WEE1 inhibitor. We collaborated with Debiopharm on the development of a combination therapy, with us sponsoring the global study, and shared all collaboration costs equally. Both parties each supplied their respective drugs and retained all commercial rights to their respective compounds, including as monotherapy or as combination therapies. The activities associated with the Debio Collaboration Agreement were coordinated by a joint steering committee, which was comprised of an equal number of representatives from both parties. Based on the terms of the Debio Collaboration Agreement, we concluded that the Debio Collaboration Agreement met the requirements of a collaboration within the guidance of ASC 808, “Collaborative Arrangements”, as both parties were active participants in the combination trial and were exposed to significant risks and rewards depending on the success of the combination trial. Accordingly, the net costs associated with the collaboration have been expensed as incurred and recognized within research and development expenses in our consolidated statement of operations and comprehensive loss.
During the three and six months ended June 30, 2025, we recognized $1.4 million and $2.7 million, respectively, in net research and development costs with regards to the Debiopharm portion of the 50/50 cost sharing terms in the Debio Collaboration Agreement, and recorded a deferred collaboration cost recovery of $3.3 million as of June 30, 2025, reflecting the acceleration of payments under the Debio Collaboration Agreement in the second quarter of 2025.
During the three and six months ended June 30, 2024, we recognized $0.9 million and $1.4 million, respectively, in net research and development costs with regards to the Debiopharm portion of the 50/50 cost sharing terms in the Debio Collaboration Agreement.
As part of the worldwide licensing agreement with Debiopharm signed on July 15, 2025, the Debio Collaboration Agreement was terminated.
Research and Development Expenses
Research and development expenses consist primarily of costs incurred for our research activities, including our drug discovery efforts and the development of our product candidates, partially offset by fully refundable Canadian research and development tax credits. We expense research and development costs as incurred, which include:
•external research and development expenses incurred under agreements with contract research organizations, or CROs, as well as investigative sites and consultants that conduct our clinical trials, preclinical studies and other scientific development services;
•employee-related expenses, including salaries, bonuses, benefits, share-based compensation, and other related costs for those employees involved in research and development efforts;
•costs related to manufacturing material for our preclinical studies and clinical trials, including fees paid to contract manufacturing organizations, or CMOs;
•laboratory supplies and research materials;
•upfront, milestone and maintenance fees incurred under license, acquisition and other third-party agreements;
•costs related to compliance with regulatory requirements; and
•facilities, depreciation, scientific advisory board and other allocated expenses, which include direct and allocated expenses for rent, maintenance of facilities and equipment, insurance, equipment and software.
Costs for certain activities are recognized based on an evaluation of the progress to completion of specific tasks using data such as information provided to us by our vendors and analyzing the progress of our studies or other services performed. Significant judgment and estimates are made in determining the accrued expense or prepaid balances at the end of any reporting period.
We characterize research and development costs incurred prior to the identification of a product candidate as discovery costs. We characterize costs incurred once a product candidate has been identified as development costs.
Our direct external research and development expenses consist primarily of fees paid to outside consultants, CROs, CMOs and research laboratories in connection with our preclinical development, process development, manufacturing and clinical development activities. Our direct external research and development expenses also include fees incurred under license, acquisition, and option agreements. We track these external research and development costs on a program-by-program basis once we have identified a product candidate.
We do not allocate employee costs, costs associated with our discovery efforts, laboratory supplies, and facilities, including depreciation or other indirect costs, to specific programs because these costs are deployed across multiple programs and, as such, are not separately classified. We use internal resources primarily to conduct our research and discovery activities as well as for managing our preclinical development, process development, manufacturing, and clinical development activities.
The following table summarizes our research and development costs:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended
June 30, |
|
|
Six Months Ended
June 30, |
|
|
|
2025 |
|
|
2024 |
|
|
2025 |
|
|
2024 |
|
|
|
(in thousands) |
|
Discovery costs |
|
|
|
|
|
|
|
|
|
|
|
|
Direct external costs |
|
$ |
259 |
|
|
$ |
1,398 |
|
|
$ |
957 |
|
|
$ |
3,124 |
|
Laboratory supplies and research materials |
|
|
90 |
|
|
|
959 |
|
|
|
344 |
|
|
|
1,957 |
|
Personnel related costs |
|
|
243 |
|
|
|
3,232 |
|
|
|
1,750 |
|
|
|
6,418 |
|
Facilities related costs |
|
|
145 |
|
|
|
393 |
|
|
|
503 |
|
|
|
798 |
|
Other costs |
|
|
65 |
|
|
|
842 |
|
|
|
643 |
|
|
|
1,754 |
|
|
|
|
802 |
|
|
|
6,824 |
|
|
|
4,197 |
|
|
|
14,051 |
|
Development costs |
|
|
|
|
|
|
|
|
|
|
|
|
Direct external costs |
|
|
|
|
|
|
|
|
|
|
|
|
Camonsertib program* |
|
|
1,848 |
|
|
|
3,961 |
|
|
|
4,114 |
|
|
|
7,941 |
|
Lunresertib program* |
|
|
3,999 |
|
|
|
7,660 |
|
|
|
8,274 |
|
|
|
15,767 |
|
RP-1664 program |
|
|
1,579 |
|
|
|
1,412 |
|
|
|
2,921 |
|
|
|
3,008 |
|
RP-3467 and Polθ program |
|
|
1,541 |
|
|
|
773 |
|
|
|
2,638 |
|
|
|
2,328 |
|
Personnel related costs |
|
|
5,070 |
|
|
|
9,186 |
|
|
|
13,233 |
|
|
|
18,845 |
|
Facilities related costs |
|
|
233 |
|
|
|
213 |
|
|
|
480 |
|
|
|
421 |
|
Other costs* |
|
|
695 |
|
|
|
1,186 |
|
|
|
1,584 |
|
|
|
2,618 |
|
Debiopharm development cost reimbursement |
|
|
(1,413 |
) |
|
|
(880 |
) |
|
|
(2,682 |
) |
|
|
(1,380 |
) |
|
|
|
13,552 |
|
|
|
23,511 |
|
|
|
30,562 |
|
|
|
49,548 |
|
R&D tax credits |
|
|
(71 |
) |
|
|
(260 |
) |
|
|
(206 |
) |
|
|
(554 |
) |
Total research and development costs |
|
$ |
14,283 |
|
|
$ |
30,075 |
|
|
$ |
34,553 |
|
|
$ |
63,045 |
|
*Certain amounts have been reclassified for presentation purposes.
The successful development of our product candidates is highly uncertain. As reflected in our financials for the quarter ended June 30, 2025, our research and development expenses have decreased as a result of the cost savings initiatives we implemented in connection with strategic re-prioritization activities implemented in August 2024 and in the first quarter of 2025 and the out-licensing of our early-stage discovery platforms to DCx in the second quarter of 2025. We expect our research and development expenses to continue to decrease going forward due to the out-licensing of our lunresertib program to Debiopharm in July 2025. We cannot determine with certainty the duration, or the completion costs of current or future preclinical studies and clinical trials of our product candidates due to the inherently unpredictable nature of preclinical and clinical development. Clinical and preclinical development timelines, the probability of success and development costs can differ materially from expectations. We anticipate that we will make determinations as to which product candidates to pursue and how much funding to direct to each product candidate on an ongoing basis in response to the results of ongoing and future preclinical studies and clinical trials, regulatory developments, and our ongoing assessments as to each product candidate’s commercial potential. We will need to raise substantial additional capital in the future. Our clinical development costs are expected to fluctuate significantly, particularly due to the numerous risks and uncertainties associated with developing product candidates, including the uncertainty of:
•the scope, rate of progress, and expenses of our ongoing preclinical studies, clinical trials and other research and development activities;
•establishing an appropriate safety profile;
•successful enrollment in and completion of clinical trials;
•whether our product candidates show safety and efficacy in our clinical trials;
•receipt of marketing approvals from applicable regulatory authorities;
•establishing commercial manufacturing capabilities or making arrangements with third-party manufacturers;
•obtaining and maintaining patent and trade secret protection and regulatory exclusivity for our product candidates;
•commercializing product candidates, if and when approved, whether alone or in collaboration with others; and
•continued acceptable safety profile of products following any regulatory approval.
Any changes in the outcome of any of these variables with respect to the development of our product candidates in preclinical and clinical development could mean a significant change in the costs and timing associated with the development of these product candidates. We may never succeed in achieving regulatory approval for any of our product candidates. We may obtain unexpected results from our clinical trials. We may elect to discontinue, delay or modify clinical trials of some product candidates or focus on other product candidates. For example, if the U.S. Food and Drug Administration, or FDA, the European Medicines Agency, or EMA, or another regulatory authority were to delay our planned start of clinical trials or require us to conduct clinical trials or other testing beyond those that we currently expect or if we experience significant delays in enrollment in any of our ongoing and planned clinical trials, we could be required to expend significant additional financial resources and time on the completion of clinical development of that product candidate.
General and Administrative Expenses
General and administrative expense consists primarily of employee related costs, including salaries, bonuses, benefits, share-based compensation and other related costs, as well as expenses for outside professional services, including legal, accounting and audit services and other consulting fees, rent expense, directors and officers insurance expenses, investor relations expenses and other general administrative expenses.
We anticipate that our general and administrative expenses may increase in the future as we explore partnering alternatives for our portfolio, including potential legal, accounting and advisory expenses and other related charges. Subject to our strategic review process, we also anticipate that we will continue to incur significant accounting, audit, legal, regulatory, compliance and directors’ and officers’ insurance costs, as well as investor relations expenses.
Restructuring Expenses
In August 2024, we announced a strategic re-prioritization of our research and development activities to focus our efforts on the advancement of our portfolio of clinical-stage oncology programs. As part of this strategic refocus, we reduced our overall workforce by approximately 25%, with a majority of the headcount reductions from our preclinical group.
In the first quarter of 2025, we announced a re-alignment of resources and a re-prioritization of our clinical portfolio to focus on the continued advancement of our Phase 1 clinical programs, RP-3467 and RP-1664. We also approved a phased reorganization plan
pursuant to which we expect to reduce our workforce by approximately 75% by the fourth quarter of 2025. As a result of this initiative, we accelerated the depreciation of our laboratory equipment by $1.0 million and $1.9 million for the three and six months ended June 30, 2025, respectively, reflecting a shorter estimated remaining useful life for the equipment.
For the three and six months ended June 30, 2025, we incurred approximately $3.4 million and $6.6 million, respectively, in costs as part of its restructuring efforts (nil for the three and six months ended June 30, 2024), comprised of $2.1 million in severance and termination benefits and $1.0 million in accelerated depreciation expense, as well as $0.3 million in other restructuring charges for the three months ended June 30, 2025, and comprised of $4.4 million in severance and termination benefits and $1.9 million in accelerated depreciation expense, as well as $0.3 million in other restructuring charges for the six months ended June 30, 2025.
Other Income (Expense), Net
Other income (expense), net consists primarily of realized and unrealized gains and losses on foreign exchange, interest income earned on cash and cash equivalents and marketable securities, and other expenses such as interest and bank charges.
Realized and unrealized gains and losses on foreign exchange consist of realized and unrealized gains and losses from holding cash and foreign currency denominated other receivables, accounts payable, accrued expenses and other current liabilities as well as operating lease liabilities.
Results of Operations
Comparison of the Three Months Ended June 30, 2025 and 2024
The following table summarizes our results of operations for the three months ended June 30, 2025 and 2024:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended
June 30, |
|
|
|
|
|
|
2025 |
|
|
2024 |
|
|
Change |
|
|
|
(in thousands) |
|
Revenue: |
|
|
|
|
|
|
|
|
|
Collaboration agreements |
|
$ |
250 |
|
|
$ |
1,073 |
|
|
$ |
(823 |
) |
Operating expenses: |
|
|
|
|
|
|
|
|
|
Research and development, net of tax credits |
|
|
14,283 |
|
|
|
30,075 |
|
|
|
(15,792 |
) |
General and administrative |
|
|
6,029 |
|
|
|
8,317 |
|
|
|
(2,288 |
) |
Restructuring |
|
|
3,384 |
|
|
|
— |
|
|
|
3,384 |
|
Total operating expenses |
|
|
23,696 |
|
|
|
38,392 |
|
|
|
(14,696 |
) |
Gain on sale of technology and other assets |
|
|
5,666 |
|
|
|
— |
|
|
|
5,666 |
|
Loss from operations |
|
|
(17,780 |
) |
|
|
(37,319 |
) |
|
|
19,539 |
|
Other income (expense), net |
|
|
|
|
|
|
|
|
|
Realized and unrealized gain on foreign exchange |
|
|
66 |
|
|
|
6 |
|
|
|
60 |
|
Interest income |
|
|
1,236 |
|
|
|
2,894 |
|
|
|
(1,658 |
) |
Other expense, net |
|
|
(18 |
) |
|
|
(29 |
) |
|
|
11 |
|
Total other income, net |
|
|
1,284 |
|
|
|
2,871 |
|
|
|
(1,587 |
) |
Loss before income taxes |
|
|
(16,496 |
) |
|
|
(34,448 |
) |
|
|
17,952 |
|
Income tax expense |
|
|
(248 |
) |
|
|
(326 |
) |
|
|
78 |
|
Net loss |
|
$ |
(16,744 |
) |
|
$ |
(34,774 |
) |
|
$ |
18,030 |
|
Revenue
Revenue was $0.3 million for the three months ended June 30, 2025, compared to $1.1 million for the three months ended June 30, 2024. The decrease of $0.8 million was due to:
•a $1.1 million decrease in revenue recognized under our collaboration and license agreement with Roche, which was terminated in May 2024; and
•a $0.3 million increase in revenue recognized under our collaboration and license agreement with Bristol-Myers Squibb as a result of the exercise of an additional option for a druggable target.
Research and Development Expenses, Net of Tax Credits
Research and development expenses were $14.3 million for the three months ended June 30, 2025, compared to $30.1 million for the three months ended June 30, 2024. The decrease of $15.8 million was due to:
•a $7.1 million decrease in personnel-related costs;
•a $3.7 million decrease in direct external costs of the lunresertib program as a result of the termination of the Phase 1 Magnetic and Minotaur clinical trials;
•a $2.1 million decrease in direct external costs of the camonsertib program as a result of the ongoing termination activities of the Phase 1/2 TRESR and ATTACC clinical trials;
•a $2.0 million decrease in other direct external costs related to discovery programs and other research and material expenses;
•a $1.2 million decrease in other R&D expenses, net of R&D tax credits;
•a $0.8 million increase in direct external costs for the RP-3467 program; and
•a $0.5 million increase in the Debiopharm development cost reimbursement.
General and Administrative Expenses
General and administrative expenses were $6.0 million for the three months ended June 30, 2025, compared to $8.3 million for the three months ended June 30, 2024. The decrease of $2.3 million in general and administrative expenses consisted of:
•a $2.3 million decrease in personnel-related costs;
•a $0.4 million decrease in other general and administrative expenses mainly due to lower IT related costs; and
•a $0.4 million increase in professional costs associated with higher legal fees in relation to out-licensing agreements and restructuring initiatives.
Restructuring Expenses
Restructuring expenses were $3.4 million and nil for the three months ended June 30, 2025 and 2024, respectively, as a result of costs incurred as part of our restructuring efforts announced in the first quarter of 2025. Changes in the second quarter of 2025 were comprised of $2.1 million in severance and termination benefits, $1.0 million in accelerated depreciation expense and $0.3 million in other restructuring charges.
Gain on Sale of Technology and Other Assets
On May 1, 2025, we out-licensed our early-stage discovery platforms, including certain platform and program intellectual property, to DCx. Under the terms of the out-licensing agreement, we received a $1.0 million upfront payment and expect to receive near-term payments of $3.0 million. In addition, we received a 9.99% equity position in DCx, including certain dilution protection rights. We are eligible to receive potential future out-licensing, clinical and commercial milestone payments, as well as low-single digit tiered sales royalties for the development of certain products by DCx. Additionally, DCx retained some of our preclinical research employees.
We determined that the assets, rights and employees transferred to DCx constitute a business as defined in ASC 805, Business Combinations and as such, we accounted for the disposal by applying the derecognition guidance in ASC 810, Consolidation, which requires that a gain or loss be recognized for the difference between the carrying value of the assets sold and the fair value of the consideration received or receivable. The total fair value of the consideration received or receivable was determined to be $6.2 million, reflecting the $1.0 million upfront payment received, the $3.0 million cash consideration receivable in the near-term, the $1.6 million equity position in DCx, and the $0.6 million dilution protection rights. The carrying value of assets disposed of as of May 1, 2025 was $0.5 million, reflecting prepaid expenses for R&D services transferred to DCx. All equipment sold and intellectual property out-licensed to DCx had no carrying value as of May 1, 2025. In connection with the disposal, we recognized a gain on sale of technology and other assets in the amount of $5.7 million in the second quarter of 2025.
Milestones and sales royalties were determined to be contingent consideration which is expected to be recognized when amounts are probable and estimable in accordance with ASC 450, Contingencies. No amounts were recognized as of June 30, 2025.
Other Income (Expense), Net
Other income, net was $1.3 million and $2.9 million for the three months ended June 30, 2025 and 2024, respectively. The decrease of $1.6 million was primarily attributable to lower sums in cash and cash equivalents and marketable securities.
Income Tax
Income tax expense was $0.2 million for the three months ended June 30, 2025, compared to $0.3 million for the three months ended June 30, 2024. The decrease of $0.1 million in income tax expense was primarily due to lower taxable income resulting from the re-alignment of resources and reductions in workforce implemented.
Comparison of the Six Months Ended June 30, 2025 and 2024
The following table summarizes our results of operations for the six months ended June 30, 2025 and 2024:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Six Months Ended
June 30, |
|
|
|
|
|
|
2025 |
|
|
2024 |
|
|
Change |
|
|
|
(in thousands) |
|
Revenue: |
|
|
|
|
|
|
|
|
|
Collaboration agreements |
|
$ |
250 |
|
|
$ |
53,477 |
|
|
$ |
(53,227 |
) |
Operating expenses: |
|
|
|
|
|
|
|
|
|
Research and development, net of tax credits |
|
|
34,553 |
|
|
|
63,045 |
|
|
|
(28,492 |
) |
General and administrative |
|
|
13,681 |
|
|
|
16,935 |
|
|
|
(3,254 |
) |
Restructuring |
|
|
6,649 |
|
|
|
— |
|
|
|
6,649 |
|
Total operating expenses |
|
|
54,883 |
|
|
|
79,980 |
|
|
|
(25,097 |
) |
Gain on sale of technology and other assets |
|
|
5,666 |
|
|
|
— |
|
|
|
5,666 |
|
Loss from operations |
|
|
(48,967 |
) |
|
|
(26,503 |
) |
|
|
(22,464 |
) |
Other income (expense), net |
|
|
|
|
|
|
|
|
|
Realized and unrealized gain on foreign exchange |
|
|
64 |
|
|
|
37 |
|
|
|
27 |
|
Interest income |
|
|
2,774 |
|
|
|
5,862 |
|
|
|
(3,088 |
) |
Other expense, net |
|
|
(40 |
) |
|
|
(53 |
) |
|
|
13 |
|
Total other income, net |
|
|
2,798 |
|
|
|
5,846 |
|
|
|
(3,048 |
) |
Loss before income taxes |
|
|
(46,169 |
) |
|
|
(20,657 |
) |
|
|
(25,512 |
) |
Income tax expense |
|
|
(618 |
) |
|
|
(955 |
) |
|
|
337 |
|
Net loss |
|
$ |
(46,787 |
) |
|
$ |
(21,612 |
) |
|
$ |
(25,175 |
) |
Revenue
Revenue was $0.3 million for the six months ended June 30, 2025, compared to $53.5 million for the six months ended June 30, 2024. The decrease of $53.2 million was due to:
•a $50.9 million decrease in revenue recognized under our collaboration and license agreement with Roche, which was terminated in May 2024; and
•a $2.3 million decrease in revenue recognized under our collaboration and license agreement with Bristol-Myers Squibb, which collaboration term ended in November 2023.
Research and Development Expenses, Net of Tax Credits
Research and development expenses were $34.6 million for the six months ended June 30, 2025, compared to $63.1 million for the six months ended June 30, 2024. The decrease of $28.5 million was due to:
•a $10.3 million decrease in personnel-related costs;
•a $7.5 million decrease in direct external costs of the lunresertib program as a result of the termination of the Phase 1 Magnetic and Minotaur clinical trials;
•a $3.8 million decrease in direct external costs of the camonsertib program as a result of the ongoing termination activities of the Phase 1/2 TRESR and ATTACC clinical trials;
•a $3.8 million decrease in other direct external costs related to discovery programs and other research and material expenses;
•a $1.8 million decrease in other R&D expenses, net of R&D tax; and
•a $1.3 million increase in the Debiopharm development cost reimbursement.
General and Administrative Expenses
General and administrative expenses were $13.7 million for the six months ended June 30, 2025, compared to $16.9 million for the six months ended June 30, 2024. The decrease of $3.2 million in general and administrative expenses consisted of:
•a $3.4 million decrease in personnel-related costs;
•a $0.8 million decrease in other general and administrative expenses mainly due to lower IT related costs and lower D&O insurance premium; and
•a $1.0 million increase in professional costs associated to higher legal fees in relation to out-licensing agreements and restructuring initiatives.
Restructuring Expenses
Restructuring expenses were $6.6 million and nil for the six months ended June 30, 2025 and 2024, respectively, as a result of costs incurred as part of our restructuring efforts announced in the first quarter of 2025, comprised of $4.4 million in severance and termination benefits, $1.9 million in accelerated depreciation expense and $0.3 million in other restructuring charges.
Gain on Sale of Technology and Other Assets
Pursuant to the out-licensing agreement entered into with DCx on May 1, 2025, we recognized a gain on sale of technology and other assets in the amount of $5.7 million in the first half of 2025.
Other Income (Expense), Net
Other income, net was $2.8 million and $5.8 million for the six months ended June 30, 2025 and 2024, respectively. The decrease of $3.0 million was primarily attributable to lower sums in cash and cash equivalents and marketable securities.
Income Tax
Income tax expense was $0.6 million for the six months ended June 30, 2025, compared to $1.0 million for the six months ended June 30, 2024. The decrease of $0.4 million in income tax expense was primarily due to lower taxable income resulting from the re-alignment of resources and reductions in workforce implemented.
Liquidity and Capital Resources
Since our inception, we have not recognized any revenue from product sales and have incurred operating losses and negative cash flows from our operations. We have not yet commercialized any product and we do not expect to generate revenue from sales of any products for several years, if at all. We have funded our operations to date with proceeds received from equity financings, including net proceeds of $232.0 million from our IPO in June 2020 and net proceeds of $94.3 million from a follow-on offering in November 2021. We have also received initial upfront and additional payments of approximately $244.1 million in the aggregate from collaboration and license agreements and the proceeds from the sale of technology and other assets.
In November 2024, we entered into a Common Shares Sale Agreement, or the 2024 Sales Agreement, with TD Securities (USA) LLC, pursuant to which we may sell up to $100.0 million in common shares. We have not issued or sold shares under the 2024 Sales Agreement.
In August 2024, we announced a strategic re-prioritization of our research and development activities to focus our efforts on the advancement of our portfolio of clinical-stage oncology programs. As part of this strategic refocus, we reduced our overall workforce by approximately 25%, with a majority of the headcount reductions from our preclinical group. Furthermore, in January 2025, we announced a re-alignment of resources and a re-prioritization of our clinical portfolio to focus on the continued advancement of our Phase 1 clinical programs, RP-3467 and RP-1664, and in February 2025 we approved a phased reduction of our workforce by 75% by the fourth quarter of 2025. We also announced our intention to seek partnering opportunities across our portfolio.
Beginning in 2022, the Tax Cuts and Jobs Act of 2017 eliminated the option to deduct certain U.S.-based research and development expenditures in the current fiscal year and required taxpayers to amortize them over five years pursuant to Section 174 of the Internal Revenue Code of 1986, as amended, or IRC. This provision increased our 2023 and 2022 cash payments of income taxes significantly as compared to 2021 in compliance with IRC Section 174. In September 2023, new interim guidance was issued by the Department of Treasury and the Internal Revenue Service on IRC Section 174 that supports the deduction of such expenses. An income tax receivable in the amount of $11.0 million as of June 30, 2025, reflects the overpayment of tax installments by our U.S. subsidiary. Any changes to tax legislation may materially affect our cash flows. Changes in our tax provisions or an increase in our tax liabilities, whether due to changes in applicable laws and regulations or our interpretation or application thereof, could have a material adverse effect on our financial position, results of operations and/or cash flows.
We expect to incur significant expenses and operating losses for the foreseeable future. As of June 30, 2025, our cash and cash equivalents and marketable securities on hand was $109.5 million. Taking into account the anticipated cost savings associated with the announced re-alignment of resources, reduction in workforce and out-licensing transactions with Debiopharm and DCx, and subject to our strategic review process, we believe that our cash, cash equivalents, and marketable securities will be sufficient to fund our anticipated operating and capital expenditure requirements through 2027. We have based this estimate on assumptions that may prove to be wrong, and we could exhaust our capital resources sooner than we expect.
Because of the numerous risks and uncertainties associated with research, development, and commercialization of our product candidates, we are unable to estimate the exact amount of our working capital requirements. Our future capital requirements will depend on many factors, including:
•the outcome of our ongoing exploration and review of strategic alternatives, including to the extent we identify and enter into any potential strategic transactions;
•the initiation, timing, costs, progress and results of our product candidates, including our ongoing clinical trials of RP-3467 and RP-1664;
•the timing and amount of milestone and royalty payments that we are required to make or eligible to receive under our current or future collaboration agreements;
•the outcome, timing and cost of meeting regulatory requirements established by the FDA, EMA and other regulatory authorities;
•the costs and timing of future commercialization activities, including product manufacturing, marketing, sales and distribution, for any of our product candidates for which we or our collaborators receive marketing approval;
•the cost of expanding, maintaining and enforcing our intellectual property portfolio, including filing, prosecuting, defending and enforcing our patent claims and other intellectual property rights;
•the cost of defending potential intellectual property disputes, including patent infringement actions brought by third parties against us or any of our product candidates;
•the effect of competing technological and market developments;
•the cost and timing of completion of commercial-scale manufacturing activities;
•the extent to which we partner our programs, acquire or in-license other product candidates and technologies or enter into additional strategic collaborations;
•the revenue, if any, received from commercial sales of RP-3467, RP-1664, lunresertib and any future product candidates for which we or our collaborators receive marketing approval; and
•the costs of operating as a public company.
Until such time, if ever, as we can generate substantial product revenues to support our cost structure, we expect to finance our cash needs through a combination of equity offerings, debt financings, collaborations and other potential transactions related to our evaluation of strategic alternatives. To the extent that we raise additional capital through the sale of equity or convertible debt securities, the ownership interest of our shareholders will be or could be diluted, and the terms of these securities may include liquidation or other preferences that adversely affect the rights of our common shareholders. Debt financing and equity financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making capital expenditures or declaring dividends. If we raise funds through collaborations, or other similar arrangements with third parties, we may have to relinquish valuable rights to our technologies, future revenue streams, research programs or product candidates or grant licenses on terms that may not be favorable to us and/or may reduce the value of our common shares. If we are unable to raise additional funds through equity or debt financings when needed, we may be required to delay, limit, reduce or terminate our product development
or future commercialization efforts or grant rights to develop and market our product candidates even if we would otherwise prefer to develop and market such product candidates ourselves.
Cash Flows
Comparison of the Six Months Ended June 30, 2025 and 2024
The following table summarizes our cash flows for each of the periods presented:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Six Months Ended
June 30, |
|
|
|
|
|
|
2025 |
|
|
2024 |
|
|
Change |
|
|
|
(in thousands) |
|
Net cash used in by operating activities |
|
$ |
(45,475 |
) |
|
$ |
(18,582 |
) |
|
$ |
(26,893 |
) |
Net cash provided by (used in) investing activities |
|
|
28,206 |
|
|
|
(13,198 |
) |
|
|
41,404 |
|
Net cash provided by financing activities |
|
|
79 |
|
|
|
375 |
|
|
|
(296 |
) |
Effect of exchange rate fluctuations on cash held |
|
|
129 |
|
|
|
(43 |
) |
|
|
172 |
|
Net Decrease In Cash And Cash Equivalents |
|
$ |
(17,061 |
) |
|
$ |
(31,448 |
) |
|
$ |
14,387 |
|
Operating Activities
Net cash used in operating activities was $45.5 million for the six months ended June 30, 2025, reflecting a net loss of $46.8 million, a net change of $1.5 million in our net operating assets, offset by a net change in non-cash charges of $2.8 million. The net change in our net operating assets was mainly due to a decrease of $8.6 million in accounts payable and accrued expenses and operating lease liability offset by an increase of $7.1 million in deferred collaboration cost recovery, prepaid expenses and other current and non current assets. The change in non-cash charges primarily consist of an increase of $9.5 million in share-based compensation for option and restricted share unit grants to employees, as well as depreciation expense, including accelerated depreciation of our laboratory equipment, and non-cash lease expense offset by $6.7 million in gain on sale of technology and other assets and net accretion of marketable securities.
Net cash used in operating activities was $18.6 million for the six months ended June 30, 2024, reflecting a net loss of $21.6 million, a net change of $9.2 million in our net operating assets, offset by non-cash charges of $12.2 million. The non-cash charges primarily consist of share-based compensation for option and restricted share unit grants to employees, as well as depreciation expense, and non-cash lease expense offset by the net accretion of marketable securities. The change in our net operating assets was due to decreases of $12.0 million in deferred revenue, $1.7 million in accrued expenses and other current liabilities, $1.1 million in operating lease liability and $1.0 million in prepaid expenses, offset by increases of $0.9 million in other current receivables, $0.9 million in income taxes and $4.8 million in accounts payable.
The $26.9 million decrease in cash used in operating activities for the six months ended June 30, 2025 compared to the six months ended June 30, 2024 is primarily due to the non-recurrence of the $40.0 million milestone payment from Roche in the first quarter of 2024 offset by the decrease in our research and development expenses due to the re-alignment of resources and re-prioritization of our clinical portfolio announced in the first quarter of 2025.
Investing Activities
Net cash provided by investing activities was $28.2 million for the six months ended June 30, 2025 and resulted primarily from proceeds on maturities of marketable securities of $73.7 million and proceeds on the disposal of assets of $1.0 million offset by the purchases of marketable securities of $46.5 million.
Net cash used in investing activities was $13.2 million for the six months ended June 30, 2024 and resulted primarily from purchases of marketable securities of $102.2 million offset by the proceeds on maturities of marketable securities of $89.0 million.
Financing Activities
Net cash provided by financing activities was $0.1 million and $0.4 million for the six months ended June 30, 2025 and 2024, respectively, consisting primarily of net proceeds from the issuance of common shares under the ESPP.
Material Cash Requirements
There were no material changes to our material cash requirements during the six months ended June 30, 2025, from those described under “Management’s Discussion and Analysis of Financial Condition and Results of Operations” in the Annual Report.
Critical Accounting Estimates
This management’s discussion and analysis is based on our unaudited condensed consolidated financial statements, which have been prepared in accordance with U.S. generally accepted accounting principles. The preparation of these unaudited condensed consolidated financial statements requires us to make judgments and estimates that affect the reported amounts of assets and liabilities, the disclosure of contingent assets and liabilities at the date of the unaudited condensed consolidated financial statements and the reported amounts of expenses during the reported periods. We base our estimates on historical experience, known trends and events, and various other factors that we believe to be reasonable under the circumstances. Actual results may differ from these estimates under different assumptions or conditions. On an ongoing basis, we evaluate our judgments and estimates in light of changes in circumstances, facts, and experience. The effects of material revisions in estimates, if any, will be reflected in the consolidated financial statements prospectively from the date of change in estimates.
There have been no significant changes to our critical accounting estimates from those described in “Management’s Discussion and Analysis of Financial Condition and Results of Operations” included in the Annual Report.
Recent Accounting Pronouncements
See Note 2 to our unaudited condensed consolidated financial statements included in this Quarterly Report for a description of recent issued accounting pronouncements not yet adopted.
Item 3. Quantitative and Qualitative Disclosures About Market Risk.
Under SEC rules and regulations, because we are considered to be a “smaller reporting company”, we are not required to provide the information required by this item in this Quarterly Report.
Item 4. Controls and Procedures.
Evaluation of Disclosure Controls and Procedures
We maintain “disclosure controls and procedures,” as defined in Rule 13a-15(e) and Rule 15d-15(e) under the Securities Exchange Act of 1934, as amended, or the Exchange Act, that are designed to ensure that information required to be disclosed by a company in the reports that it files or submits under the Exchange Act is recorded, processed, summarized and reported, within the time periods specified in the SEC’s rules and forms. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed by a company in the reports that it files or submits under the Exchange Act is accumulated and communicated to our management, including our principal executive and principal financial officers, as appropriate to allow timely decisions regarding required disclosure.
Our management, with the participation of our Chief Executive Officer/Chief Financial Officer, has evaluated the effectiveness of our disclosure controls and procedures as of June 30, 2025. Based upon that evaluation, our Chief Executive Officer/Chief Financial Officer has concluded that, as of such date, our disclosure controls and procedures are effective.
Changes in Internal Control over Financial Reporting
There were no changes in our internal control over financial reporting (as defined in Rule 13a-15(f) and 15d-15(f) under the Exchange Act that occurred during the period covered by this Quarterly Report that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Inherent Limitations on Effectiveness of Controls
Our disclosure controls and procedures and internal control over financial reporting are designed to provide reasonable assurance of achieving the desired control objectives. Our management recognizes that any control system, no matter how well designed and operated, is based upon certain judgments and assumptions and cannot provide absolute assurance that its objectives will be met. Similarly, an evaluation of controls cannot provide absolute assurance that misstatements due to error or fraud will not occur or that all control issues and instances of fraud, if any, have been detected.
PART II—OTHER INFORMATION
Item 1. Legal Proceedings.
From time to time, we may become involved in legal proceedings arising in the ordinary course of our business. We are not currently a party to any material legal proceedings, and we are not aware of any pending or threatened legal proceeding against us that we believe could have an adverse effect on our business, operating results or financial condition.
Item 1A. Risk Factors.
Investing in our common shares involves a high degree of risk. In addition to the other information set forth in this Quarterly Report on Form 10-Q, you should carefully consider the risks described in the Annual Report, including the disclosure therein under Part I, Item 1A, “Risk Factors,” before deciding whether to invest in our common shares. These are not the only risks facing our business. Other risks and uncertainties that we are not currently aware of or that we currently consider immaterial also may materially adversely affect our business, financial condition and future results. Risks we have identified but currently consider immaterial could still also materially adversely affect our business, financial condition and future results of operations if our assumptions about those risks are incorrect or if circumstances change.
There were no material changes during the period covered in this Quarterly Report to the risk factors previously disclosed in Part I, Item 1A of the Annual Report, except as follows:
International trade policies, including tariffs, sanctions and trade barriers may adversely affect our business, financial condition and results of operations.
We operate in a global economy, and our business depends on a global supply chain for the development, manufacturing, and distribution of our clinical trial drug products. There is inherent risk, based on the complex relationships among the U.S. and the countries in which we conduct our business, that political, diplomatic, and national security factors can lead to global trade restrictions and changes in trade policies and export regulations that may adversely affect our business and operations. The current international trade and regulatory environment is subject to significant ongoing uncertainty.
We source our active pharmaceutical ingredients (APIs) and precursor chemicals from international suppliers, with significant reliance on foreign manufacturers, including China. The ongoing trade tensions between the United States and China have resulted in multiple rounds of tariffs affecting pharmaceutical ingredients, manufacturing equipment, and related supplies. Tariffs on our API chain directly or indirectly linked to Chinese manufacturing may significantly increase our manufacturing costs for our clinical trial drug products. Should the current tariffs on China hold or additional tariffs be imposed specifically targeting Chinese pharmaceutical imports, our manufacturing costs could rise significantly.
Current or future tariffs may result in increased research and development expenses, including with respect to increased costs associated with APIs and raw materials. Trade restrictions affecting the import of materials necessary for clinical trials could result in delays to our development timelines. Increased development costs and extended development timelines could place us at a competitive disadvantage compared to companies operating in regions with more favorable trade relationships and could reduce investor confidence and negatively impact our business, results of operations and, financial condition.
Trade disputes, tariffs, restrictions and other political tensions between the United States and other countries may also exacerbate unfavorable macroeconomic conditions including inflationary pressures, foreign exchange volatility, financial market instability, and economic recessions or downturns. The ultimate impact of current or future tariffs and trade restrictions remains uncertain and could materially and adversely affect our business and financial condition. While we monitor these risks, any prolonged economic downturn or escalation in trade tensions could materially and adversely affect our business, ability to access the capital markets or other financing sources, results of operations and financial condition. In addition, tariffs and other trade developments have and may continue to heighten the risks related to the other risk factors described elsewhere in our Annual report on Form 10-K.
Enacted and future healthcare legislation may increase the difficulty and cost for us to progress our clinical programs and obtain marketing approval of and commercialize our product candidates and may affect the prices we may set.
In the United States and other jurisdictions, there have been, and we expect there will continue to be, a number of legislative and regulatory changes and proposed changes to the healthcare system that could affect our future results of operations. For example, in March 2010, the Patient Protection and Affordable Care Act, or ACA, was enacted, which substantially changed the way healthcare is financed by both governmental and private insurers.
There have been judicial, Congressional and executive branch challenges and amendments to certain aspects of the ACA. For example, on August 16, 2022, the Inflation Reduction Act of 2022, or IRA, was signed into law, which among other things, extends enhanced subsidies for individuals purchasing health insurance coverage in ACA marketplaces through plan year 2025. The IRA also eliminates the “donut-hole” under the Medicare Part D program beginning in 2025 by significantly lowering the beneficiary maximum out-of-pocket cost and through a newly established manufacturer discount program. It is possible that the ACA will be subject to judicial or Congressional challenges in the future. It is also unclear how any such challenges and additional healthcare reform measures of the second Trump administration will impact the ACA and our business.
In addition, other legislative changes have been proposed and adopted in the United States since the ACA was enacted. For example, on July 4, 2025, the annual reconciliation bill, the “One Big Beautiful Bill Act,” or OBBBA, was signed into law which is expected to reduce Medicaid spending and enrollment by implementing work requirements for some beneficiaries, capping state-directed payments, reducing federal funding, and limiting provider taxes used to fund the program. OBBBA also narrows access to ACA marketplace exchange enrollment and declines to extend the ACA enhanced advanced premium tax credits, set to expire in 2025, which, among other provisions in the law, are anticipated to reduce the number of Americans with health insurance. Additionally, in August 2011, the Budget Control Act of 2011, among other things, led to aggregate reductions of Medicare payments to providers of 2% per fiscal year. These reductions went into effect in April 2013 and, due to subsequent legislative amendments to the statute will remain in effect until 2032 unless additional action is taken by Congress. On March 11, 2021, the American Rescue Plan Act of 2021 was signed into law, which eliminated the statutory Medicaid drug rebate cap, previously set at 100% of a drug’s average manufacturer price, for single source and innovator multiple source drugs, beginning January 1, 2024. These new laws or any other similar laws introduced in the future may result in additional reductions in Medicare and other health care funding, which could negatively affect our customers and accordingly, our financial operations.
Moreover, payment methodologies may be subject to changes in healthcare legislation and regulatory initiatives. For example, CMS may develop new payment and delivery models, such as bundled payment models. In addition, recently there has been heightened governmental scrutiny over the manner in which manufacturers set prices for their marketed products, which has resulted in several U.S. Congressional inquiries and proposed and enacted federal legislation designed to, among other things, bring more transparency to drug pricing, reduce the cost of prescription drugs under Medicare, and review the relationship between pricing and manufacturer patient programs. In addition, the IRA, among other things, (1) directs HHS to negotiate the price of certain high-expenditure, single-source drugs that have been on the market for at least 7 years and biologics that have been on the market for at least 11 years covered under Medicare (the "Medicare Drug Price Negotiation Program") and (2) imposes rebates under Medicare Part B and Medicare Part D to penalize price increases that outpace inflation. The IRA permits HHS to implement many of these provisions through guidance, as opposed to regulation, for the initial years. HHS has and will continue to issue and update guidance as these programs are implemented. These provisions began to take effect progressively starting in fiscal year 2023. On August 15, 2024, HHS announced the agreed-upon reimbursement price for the first ten drugs that were subject to price negotiations, although the Medicare Drug Price Negotiation Program is currently subject to legal challenges. On January 17, 2025, HSS selected fifteen additional products covered under Part D for price negotiations in 2025. Each year thereafter more Part B and Part D products will become subject to the Medicare Drug Price Negotiation Program. Further, on December 7, 2023, an initiative to control the price of prescription drugs through the use of march-in rights under the Bayh-Dole Act was announced. On December 8, 2023, the National Institute of Standards and Technology published for comment a Draft Interagency Guidance Framework for Considering the Exercise of March-In Rights which for the first time includes the price of a product as one factor an agency can use when deciding to exercise march-in rights. While march-in rights have not previously been exercised, it is uncertain if that will continue under the new framework. It is unclear whether the models will be utilized in any health reform measures in the future.
The current Trump administration is pursuing policies to reduce regulations and expenditures across government including at HHS, the FDA, CMS and related agencies. These actions, presently directed by executive orders or memoranda from the Office of Management and Budget, may propose policy changes that create additional uncertainty for our business. These actions and proposals may, for example, include directives: (1) reducing agency workforce and cutting programs; (2) rescinding a Biden administration executive order tasking the Center for Medicare and Medicaid Innovation (“CMMI”) to consider new payment and healthcare models to limit drug spending; (3) eliminating the Biden administration’s executive order that directed HHS to establishing an AI task force and developing a strategic plan; (4) directing HHS and other agencies to lower prescription drug costs through a variety of initiatives, including by improving upon the Medicare Drug Price Negotiation Program and establishing Most-Favored-Nation pricing for pharmaceutical products; (5) imposing tariffs on imported pharmaceutical products; and (6) directing certain federal agencies to enforce existing law regarding hospital and plan price transparency and by standardizing prices across hospitals and health plans.. Additionally, in its June 2024 decision in Loper Bright Enterprises v. Raimondo (“Loper Bright”), the U.S. Supreme Court overturned the longstanding Chevron doctrine, under which courts were required to give deference to regulatory agencies’ reasonable interpretations of ambiguous federal statutes. The Loper Bright decision could result in additional legal challenges to current regulations and guidance issued by federal agencies applicable to our operations, including those issued by the FDA. Congress may introduce and ultimately pass health care related legislation that could impact the drug approval process and make changes to the Medicare Drug Price Negotiation Program created under the IRA.
We expect additional U.S. federal healthcare reform measures will be adopted in the future, any of which could limit the amounts that the U.S. federal government will pay for healthcare products and services, which could result in reduced demand for our product candidates or additional pricing pressures.
Changes to tax laws could have a material adverse effect on us and reduce net returns to our shareholders.
Our tax treatment is subject to changes in tax laws, regulations and treaties, or the interpretation thereof, as well as tax policy initiatives and reforms under consideration and the practices of tax authorities in jurisdictions in which we operate. Such changes may include (but are not limited to) the taxation of operating income, investment income, dividends received or, in the specific context of withholding tax, dividends paid.
In December 2017, the U.S. government enacted comprehensive tax legislation, the Tax Cuts and Jobs Act of 2017, or TCJA, significantly reformed the Code. As a result of this legislation, U.S.-based specified research and experimental expenditures are required to be capitalized and amortized ratably over a five-year period. Any such expenditures attributable to research conducted outside the United States must be capitalized and amortized over a 15-year period. Prior to the enactment of the TCJA, research and experimental expenditures were deductible in the year they were incurred for U.S. tax purposes.
On July 4, 2025, the One Big Beautiful Bill Act (“OBBBA”) was signed into law, introducing significant changes to U.S. federal tax law. The OBBBA includes a broad range of changes to existing U.S. tax law, including but not limited to the reinstatement of current expensing of domestic research and development costs and one hundred percent bonus depreciation for certain qualified business property. We are evaluating the impact of the OBBBA for both 2025 and future tax years.
We are unable to predict what tax reform may be proposed or enacted in the future or what effect such changes would have on our business, but such changes, to the extent they are brought into tax legislation, regulations, policies or practices, could affect our financial position and overall or effective tax rates in the future in countries where we have operations, reduce post-tax returns to our shareholders, and increase the complexity, burden and cost of tax compliance.
New income, sales, use or other tax laws, statutes, rules, regulations or ordinances could be enacted at any time, which could affect the tax treatment of our domestic and foreign earnings. Any new taxes could adversely affect our domestic and international business operations, and our business and financial performance. Further, existing tax laws, statutes, rules, regulations or ordinances could be interpreted, changed, modified or applied adversely to us. Changes in corporate tax rates, the realization of net deferred tax assets relating to our operations, the taxation of foreign earnings, and the deductibility of expenses could have a material impact on the value of our deferred tax assets, could result in significant one-time charges, and could increase our future tax expenses.
Item 2. Unregistered Sales of Equity Securities and Use of Proceeds.
(a) Recent Sales of Unregistered Securities
None.
(b) Issuer Purchases of Equity Securities
None.
Item 3. Defaults Upon Senior Securities.
None.
Item 4. Mine Safety Disclosures.
Not applicable.
Item 5. Other Information.
Trading Arrangements
During the three months ended June 30, 2025, none of our directors or officers (as defined in Rule 16a-1(f) under the Exchange Act) adopted, terminated or modified a Rule 10b5-1 trading arrangement or non-Rule 10b5-1 trading arrangement (as such terms are defined in Item 408 of Regulation S-K.
Item 6. Exhibits.
* Filed herewith.
** This certification is being furnished solely to accompany this Quarterly Report on Form 10-Q pursuant to 18 U.S.C. Section 1350, and is not being filed for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, or otherwise subject to the liability of that section, nor shall it be deemed incorporated by reference into any filing of the registrant under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended, whether made before or after the date hereof, regardless of any general incorporation language in such filing.
† Certain portions of this exhibit (indicated by asterisks) have been omitted because they are not material and would likely cause competitive harm to Repare Therapeutics Inc. if publicly disclosed.
+ Indicates management contract or compensatory plan.
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
|
|
|
|
|
|
REPARE THERAPEUTICS INC. |
|
|
|
|
Date: August 8, 2025 |
|
By: |
/s/ Steve Forte |
|
|
|
Steve Forte |
|
|
|
President, Chief Executive Officer and Chief Financial Officer (Principal Executive Officer and Principal Financial Officer) |


